Inexpensive material boosts battery capacity
Battery-powered cars offer many environmental benefits, but a car with a full tank of gasoline can travel further. By improving the energy capacity of lithium-ion batteries, a new electrode made from iron oxide nanoparticles could help electric vehicles to cover greater distances.
Developed by Zhaolin Liu of the A*STAR Institute of Materials Research and Engineering, Singapore, and Aishui Yu of Fudan University, China, and co-workers, the electrode material is inexpensive, suitable for large-scale manufacturing and can store higher charge densities than the conventional electrodes used in lithium-ion batteries.
These batteries store and release energy by shuttling lithium ions between two electrodes connected in a circuit. During charging, lithium ions escape from the cathode, which is made from materials such as lithium cobalt oxide. The ions migrate through a liquid electrolyte and into the anode, which is usually made of graphite riddled with tiny pores. When the battery discharges, the process runs in reverse, generating an electrical current between the electrodes.
Iron oxides have a much higher charging capacity than graphite, but the process is slow. Forcing lithium ions into the material also changes its volume, destroying the anode after just a few charging cycles.
Liu, Yu and team reasoned that an anode made from iron oxide nanoparticles would charge more quickly, because its pores would give ready access to lithium ions. The pores may also allow the material's structure to change as the ions pack inside.
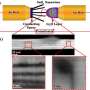
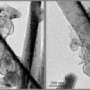
The researchers made 5-nanometer-wide particles of an iron oxide known as α-Fe2O3, simply by heating iron nitrate in water. They mixed the particles with a dust called carbon black, bound them together with polyvinylidene fluoride and coated the mixture onto copper foil to make their anodes.
During the first round of charging and discharging, the anodes showed an efficiency of 75–78%, depending on the current density used. After ten more cycles, however, the efficiency improved to 98%, almost as high as commercial lithium-ion batteries. Research by other teams suggests that during the first few cycles, the iron oxide nanoparticles are broken down until they reach an optimum size.
After 230 cycles the anode's efficiency remained at 97%, with a capacity of 1,009 milliamp hours per gram (mA h g−1 )—almost three times greater than commercial graphite anodes. The material experienced none of the degradation problems that have plagued other iron oxide anodes.
The team is now working to optimize the nanoparticle synthesis and increase the efficiency of the anode's initial charging cycles.
More information: Zhang, J., et al. Mesoporous Fe2O3 nanoparticles as high performance anode materials for lithium-ion batteries, Electrochemistry Communications 29, 17–20 (2013). dx.doi.org/10.1016/j.elecom.2013.01.002
Journal information: Electrochemistry Communications