Chemist devises optical imaging technique to unlock the mystery of memory
(Phys.org) —In the search to understand memory, Wei Min is looking at cells at the most basic level, long before the formation of neurons and synapses. The assistant professor of chemistry studies the synthesis of proteins, the building blocks of the body formed using genetic code from DNA. "We want to understand the molecular nature of memory, one of the key questions that remain in neuroscience," he says.
Proteins carry out almost every biological function, and protein synthesis is a crucial step in gene expression, determining how cells respond to pathological conditions caused by cancer, autism and the physiological stresses linked to disorders like Parkinson's and Alzheimer's. Min's lab examines the proteome (the sum of the cell's proteins), a dynamic structure tightly regulated by both production and death of proteins that ensures that the body functions normally. The formation of long-term memory is dependent on protein synthesis at a specific location and time in brain tissues.
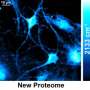
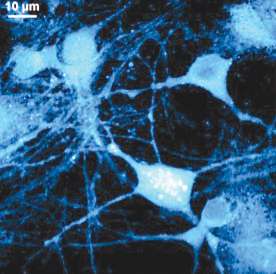
Min and his team recently developed a new imaging technique to pinpoint exactly where and when cells produce new proteins. The method is significant in that it enables scientists to create high-resolution images of newly synthesized proteins in living cells. The findings were published in the July 9 issue of the Proceedings of the National Academy of Sciences, and the research was done in collaboration with Baylor College of Medicine.
Despite extensive efforts, it is not possible yet to observe global protein (proteome) synthesis as it happens because the most widely used methods require killing cells. Min's research, however, opens the door to answering questions about the behavior of living cells because with his technique, it is possible to observe them as they perform their functions. "Instead of looking at a static picture, we are adding a new functional dimension and tool compatible with live cells," he says.
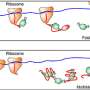
Proteins are comprised of a chain of amino acids, which are mainly made up of carbon, oxygen, hydrogen and nitrogen. In his lab in the Northwest Corner building, Min and his team replaced the hydrogen with deuterium, an isotope that is a heavier cousin of hydrogen. (Columbia Professor Harold Urey discovered deuterium in 1932, for which he won the 1934 Nobel Prize in chemistry.) Deuterium mimics the properties of hydrogen with little variation, and amino acids labeled with deuterium behave almost identically to natural amino acids. Importantly, the carbon-deuterium bond vibrates at a unique frequency different from the normal carbon-hydrogen bond.
Min's team added the deuterium-labeled amino acids to a growth medium in cell cultures, and as the deuterium-labeled amino acids were incorporated as the necessary building blocks into proteins, the researchers sought out the unique frequency to detect those carbon-deuterium bonds carried by the newly synthesized proteins.
Using a special laser-based technology called stimulated Raman scattering microscopy, they scanned a laser across the sample and created location-dependent maps of the carbon-deuterium bonds inside living cells.
"Our technique is highly sensitive, specific and compatible with living systems and doesn't require killing cells or staining," says Lu Wei, a Ph.D. student in Min's lab and lead author of the paper. Wei is currently researching where and when a new protein is produced inside brain tissues as long-term memory is formed.
Min was first intrigued by enduring neuroscience questions about memory when he was at Harvard, where he received his Ph.D. in 2008 and stayed for two years as a post-doc. A native of China who studied chemistry at Peking University in Beijing, he joined Columbia in 2010. He is a member of the Kavli Institute for Brain Science, part of Columbia's interdisciplinary neuroscience research initiative. The work on long-term memory by Nobel laureate and University Professor Eric Kandel inspired him to focus on the role of protein synthesis. "It's a cutting-edge research question and isn't yet resolved," Min says. "Our technique will help open up understanding of the many complex behaviors in learning and disease."
Journal information: Proceedings of the National Academy of Sciences
Provided by Columbia University