Core / shell photocatalyst with spatially separated cocatalysts for more efficient water splitting
Photocatalytic splitting of water uses sunlight to split water into hydrogen and oxygen. It is an environmentally friendly way to obtain hydrogen for fuel cells. In the journal Angewandte Chemie, Japanese researchers have now introduced a new method for the production of more effective photocatalysts. Their method uses tiny hollow spheres coated with different cocatalysts on the inside and the outside.
In a photocatalytic water splitting reaction, the catalyst, usually a semiconductor, captures photons. Electrons get excited and rise from the valence band to the conduction band. The electronic voids left behind in the valence band are regarded as positively charged "holes". If the electrons and holes manage to migrate to the surface of the catalyst before the opposite charges recombine, they can be transferred to water molecules and used to reduce the water to make hydrogen or oxidize it to make oxygen.
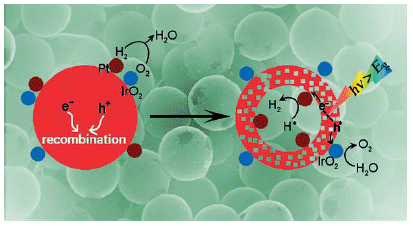
New catalyst systems are constantly being researched and developed, but their efficiency has always been found to be lacking. Theoretically, catalysts based on tantalum nitride (Ta3N5) should be especially well-suited candidates for photocatalysis with visible light. However, two main problems have hindered their successful use in practice: First, on the surface of the catalyst, the resulting products, oxygen and hydrogen, immediately react to produce water. Second, the charge separation of the electrons and holes formed in the reaction doesn't quite work correctly as they recombine too quickly.
Cocatalysts are meant to improve efficiency by capturing the electrons or holes and transferring them to the water. Precious metals like platinum can improve the individual step of reduction to hydrogen; metal oxides such as iridium and cobalt oxide catalyze the oxidation to oxygen. However, equipping photocatalysts with both types of cocatalyst has not resulted in any resounding successes.
A team led by Kazunari Domen at the University of Tokyo had a clever idea: what if both cocatalysts were not uniformly distributed over the catalyst, but instead were spatially separated? To achieve this, the researchers developed a simple method for the production of core/shell microparticles. In the first step, they coated silicon dioxide microspheres with platinum nanoparticles and then with tantalum oxide, which was converted to tantalum nitride by reaction with ammonia in the next step. The spheres were then coated with either iridium or cobalt oxide. The silicon dioxide core can be selectively dissolved, leaving behind whisper-thin, porous, hollow spheres made of tantalum nitride, coated on the inside with platinum nanoparticles and on the outside with iridium or cobalt oxide. This special construction prevents the two reaction steps from taking place in close proximity, improving the charge separation and thus the photocatalytic activity.
More information: Domen, K. Core/Shell Photocatalyst with Spatially Separated Cocatalysts for Efficient Reduction and Oxidation of Water, Angewandte Chemie International Edition. dx.doi.org/10.1002/anie.201303693
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Angewandte Chemie