Excited, but cold: Scientists unveil the secret of a reaction for prebiotic synthesis of organic matter
How is it that a complex organism evolves from a pile of dead matter? How can lifeless materials become organic molecules that are the bricks of animals and plants? Scientists have been trying to answer these questions for ages. Researchers at the Max Planck Institut für Kohlenforschung have now disclosed the secret of a reaction that has to do with the synthesis of complex organic matter before the origin of life.
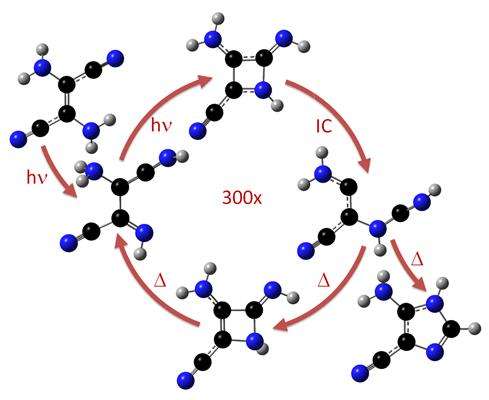
Since the 1960's it has been well known that when concentrated hydrogen cyanide (HCN) is irradiated by UV light, it forms an imidazole intermediate that is a key substance for synthesis of nucleobases and nucleotides in abiotic environment. The way how UV radiation acts in this reaction to produce complex organic matter was, however, never clarified. Dr. Mario Barbatti and his colleagues in Germany, India and Czech Republic have now shown how this process occurs via computer simulations.
Using diverse computational-chemistry methods, the team has arrived at astonishing conclusions: For example that the reaction does not take place in the hot spot created by the solar radiation. "This has nothing to do with heat, but with electrons", says Mario Barbatti.
The reaction proceeds through a series of electronically excited intermediates. The molecules get into the "electronic excited state" because of the UV radiation, which means that their electrons are distributed in a much different way than the usual. That changes the molecule's attitudes. "But this takes some time", says Mario Barbatti. They showed that the radiation energy is dissipated too fast, and because of that each reactant molecule absorbs hundreds of UV photons before it finally gets converted into the imidazole intermediate.
"This is very inefficient – and quite extraordinary", says Mario Barbatti. That is why it was quite challenging to comprehend the reaction, explains the physicist from Brazil. He and his colleagues have calculated a lot of possible intermediates, tried – and discarded most of them. Finally they found out that there is only one single pathway that is consistent with the fast energy dissipation and previous experimental observations.
But why did they work on the computer? Isn't it the case that chemical reactions are worked on in laboratories? "Some intermediates are too elusive to analyze them in the laboratory – they disappear before we may see them", Barbatti explains. Computational Chemistry allows the scientists to comprehend the reactions in a theoretical way.
"As I said before, this reaction has nothing to do with heat", says Barbatti. The transformation works in a cold environment, as in comets and in terrestrial ices, where spontaneous HCN polymerization is most expected to occur.
The team has published their results, which help to understand the role of solar radiation on the origin of life, in the recent issue of Angewandte Chemie.
More information: Boulanger, E. et al. Photochemical Steps in Prebiotic Synthesis of Purine Precursors from HCN, Angew. Chem. Int. Ed. doi: 10.1002/anie.201303246 (2013)
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Max Planck Society

















