Researchers develop unique method for creating uniform nanoparticles
(Phys.org) —University of Illinois researchers have developed unique approach for the synthesis of highly uniform icosahedral nanoparticles made of platinum. Results showed that the key factors for the shape control include fast nucleation, kinetically controlled growth, and protection from oxidation by air.
"We have developed unique approach for the synthesis of highly uniform icosahedral nanoparticles made of platinum (Pt)," explained Hong Yang, a professor of chemical and biomolecular engineering at the University of Illinois at Urbana-Champaign. "This is important both in fundamental studies—nanoscience and nanotechnology—and in applied sciences such as high performance fuel cell catalysts."
Yang's research group focuses on the synthesis and understanding structure-property relationship of nanostructured materials for applications in energy, catalysis, and biotechnology. Its paper, "Highly Uniform Platinum Icosahedra Made by the Hot Injection-Assisted GRAILS Method," was published this week in Nano Letters.
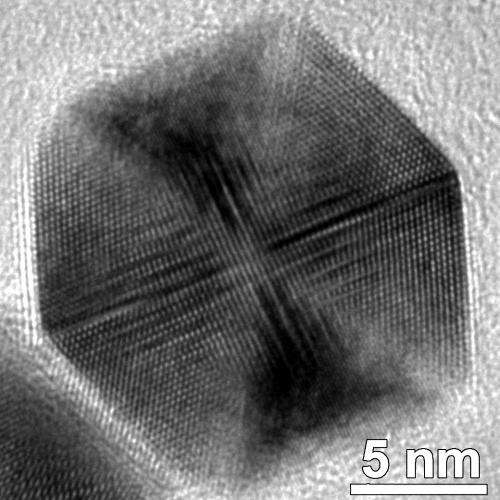
"Although polyhedral nanostructures, such as a cube, tetrahedron, octahedron, cuboctahedron, and even icosahedron, have been synthesized for several noble metals, uniform Pt icosahedra do not form readily and are rarely made," stated Wei Zhou, a visiting scholar with Yang's research group and the paper's first author.
An icosahedron crystal is a polyhedron with 20 identical equilateral triangular faces, 30 edges and 12 vertices. According to Yang, icosahedral shaped crystals can improve the catalytic activity in oxygen reduction reaction partly because of the surface strain.
"The key reaction step to improve the activity of oxygen electrode catalysts in the hydrogen fuel cell is to optimize the bond strength between Pt and absorbed oxygen-containing intermediate species," Yang said. "This allows the rapid production of water and let the intermediate react and leave the surface quickly so the catalyst site can be used again."
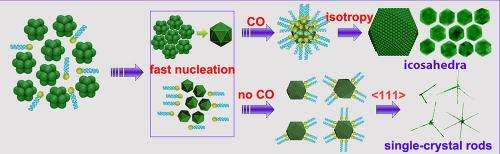
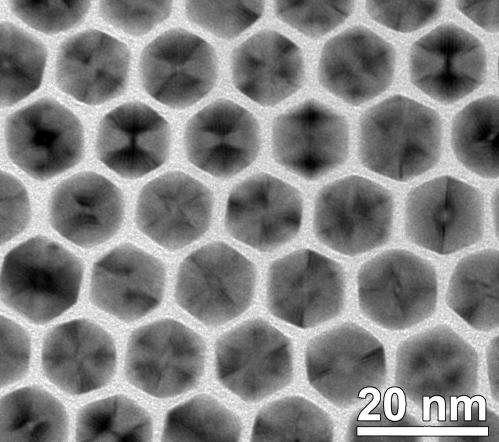
"Unlike many other forms of metal nanoparticles, an icosahedral nanocrystal is not a single crystal, but has many twin (defect) boundaries within this shape. Previous simulation data suggest that it is unstable for Pt nanoparticles to exist in this shape at about >1-2 nm and, indeed, it is uncommon for Pt nanoparticles to have this morphology." Highly uniform Pt icosahedral nanocrystals with an edge length of 8.8 nm were synthesized by Yang's research group. They were made from platinum acetylacetonate in dodecylamine and with small amount of oleic acid using a hot injection-assisted GRAILS (gas reducing agent in liquid solution) approach. In the GRAILS approach, the inclusion of CO gas greatly facilitates the formation of well-defined shapes.
"Our results showed that the key factors for the shape control include fast nucleation, kinetically controlled growth, and protection from oxidation by air," Zhou added. By adjusting these key parameters, Pt hyper-branched rods, cubes, and octapods were also obtained.
"We are currently studying why this shape is formed in our systems and how we can use this principle to produce other unusual and potentially useful Pt and its alloy nanoparticles," Yang noted. "The high purity (>95%) of the products provides the ideal model materials for studying the structure/morphology-property relationships. Such mechanistic understanding is valuable for the design of advanced, high performance metal and metal alloy catalysts."
Journal information: Nano Letters
Provided by University of Illinois at Urbana-Champaign