New technique allows scientists to directly compare catalysts' efficiency in different situations
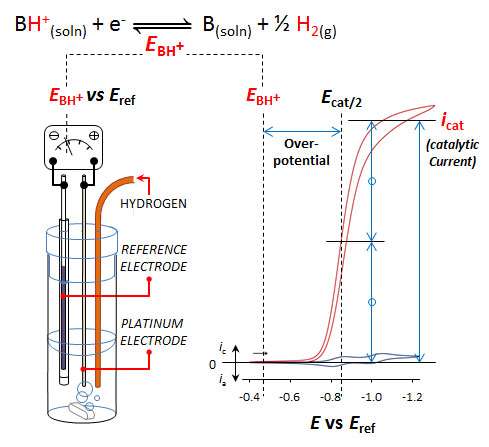
(Phys.org) —Given two catalysts for the job of turning intermittent wind or solar energy into chemical fuels, scientists chose the material that gets the job done quickly and uses the least energy. A catalyst that quickly produces fuel but uses far more energy than it stores won't get the job. Scientists could measure the wasted energy, also known as overpotential, in water but not in other liquids, until researchers at Pacific Northwest National Laboratory devised a quick, elegant technique.
"We could make some educated guesses and do back-of-the-envelope calculations as to overpotential in organic solvents, but it wasn't good enough," said Dr. R. Morris Bullock, one of the study's two authors and the Director of the Center for Molecular Electrocatalysis, an Energy Frontier Research Center.
Matching our demand for electricity with our supply requires that we learn how to store electrical energy in large amounts, cheaply and reversibly. A storage option is chemical bonds, specifically the bonds between two hydrogen atoms. Platinum metal can make that conversion happen, but it is an expensive and impractical catalyst. An inexpensive, energy-efficient and fast catalyst is needed. This study provides the scientific community with a direct method to measure a catalyst's energy efficiency.
"This research allows us to compare energy efficiencies as a function of solvent," said Dr. John Roberts, an inorganic chemist who led this study and conducted the electrochemical experiments. "Changing the solvent can make the catalyst both faster and more energy efficient. This happens because rate and energy efficiency are related to proton movement, and proton movement is related to the solvent, the catalyst, and how they interact."
In the lab, the team focused on a simple reaction that converts electrical energy into chemical bonds:
Brønsted Acid + Electrons <==> Brønsted Base + Hydrogen
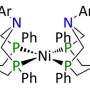
Roberts and Bullock ran the experiments in the well-known organic solvent acetonitrile, with acid, base, and hydrogen added. First, they used a pair of ordinary electrodes as a "volt meter" to find out how energetic the electrons needed to be in the ideal case. Then, they compared this ideal voltage to the voltage required for a real catalyst, in this case a nickel diphosphine complex developed by Bullock and his colleagues at the Center for Molecular Electrocatalysis. The difference in these two voltages is the first accurate measure of the overpotential for any catalyst operating in this solvent. They have demonstrated the technique with the reaction in fluorobenzene and organic solvent/water mixtures as well. "The energy efficiencies of catalyst systems interconverting electrical energy with hydrogen fuel may now be compared on an equal footing, using any solvent," said Bullock.
The team ran the experiments with different acids. They studied triethylammonium, dimethylformamidium, 2,6-dichloroanilinium, 4-cyanoanilinium, 4-bromoanilinium, and 4-anisidinium salts. In every case, the results agreed perfectly with thermodynamic calculations.
Researchers are using this direct measurement technique to determine the efficiency of other catalysts in different situations for different jobs. "This research allows us to systematically investigate what makes catalysts efficient, an important question for the jobs these catalysts can do for the Department of Energy and for industry," said Roberts.
More information: Roberts, J. and Bullock, R. 2013. Direct Determination of Equilibrium Potentials for Hydrogen Oxidation/Production by Open Circuit Potential Measurements in Acetonitrile. Inorganic Chemistry 52(7):3823-2835. DOI: 10.1021/ic302461q
Journal information: Inorganic Chemistry
Provided by Pacific Northwest National Laboratory