Detecting mirror molecules: New technique reliably tells left-handed from right-handed variant of a compound
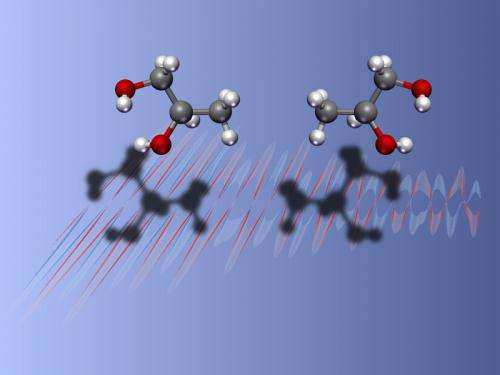
Harvard physicists have developed a novel technique that can detect molecular variants in chemical mixtures – greatly simplifying a process that is one of the most important, though time-consuming, processes in analytical chemistry.
As described in a paper in Nature, post-doctoral researcher David Patterson, Professor of Physics John Doyle and Dr. Melanie Schnell of the Center for Free-Electron Laser Science (CFEL) in Hamburg, Germany developed a system that relies on finely-tuned microwave fields to identify molecular variants apart, and to determine how much variant is in a mixture.
The ability to tell such variants apart, researchers said, is critical because many chemical compounds exist in two forms, each of which is a mirror image of the other. Such molecules are called chiral, from the ancient Greek for hand, and are often described as being either "right-handed" or "left-handed."
Knowing whether a molecule is right- or left-handed, scientists say, is important, because each type of molecule behaves differently in chemical reactions. Much of biology, for example, is predicated on the idea that amino acids are "left-handed," while sugar molecules are "right-handed."
"The 'wrong' sort of a compound can function completely differently in an organism," explains Schnell, who leads an independent Max Planck research group for structure and dynamics of molecules at CFEL. "In the best case it is just ineffective. In the worst case it is toxic."
The challenge, however, is that telling the two variants of a chiral molecule apart is no easy job.
A common way to discern between them is to shine linear polarised light through them. While one variant will turn the plane of polarisation to the left, the other will turn it to the right. The problem with that method, researchers say, is that it produces rather weak effects, and can only be used on liquid samples, and it can be difficult to use on samples that contain a mixture of many different species.
"It's an extremely common situation to have a mixture – say a blood sample, or something from a complex chemical process - that contains a left-handed version of some compounds and a right handed version others – for example, left handed alanine along with right handed citric acid. Optical polarimetery really struggles with such a situation – if there's more than about 3 compounds, it's pretty hopeless. We hope our technique will provide a tool which can produce a complete analysis of such a mixture".
In contrast, the method developed by Patterson, Doyle and Schnell, by comparison, relies on what is called the electric dipole moment of each molecule, or the way each interacts with an external electric field. As a consequence of their mirror-image construction, molecules rotate in opposite directions when certain microwave fields are applied – and this results in a signature which tells if the molecules are left or right handed.
To measure the dipole moment of molecules, the team used microwaves.
Researchers fed a gaseous sample into a chamber, then cooled it to -226 degrees Celsius. As the cold gas interacted with a precisely-tuned microwave fieldwhich caused the molecules to spin and give off their own microwave radiation. By monitoring those emissions, researchers are able to tell whether the molecules are right- or left-handed.
The researchers tested their method using the organic compound 1,2-propanediol, and were able to reliably differentiate between the two variants, but also determine the ratio of variants in a mixture by finely-tuning the microwave frequency.
"We can soon measure mixtures of different compounds and determine the enantiomer ratios of each," explains Schnell. In a next step the researchers plan to apply the technique in a broadband spectrometer at CFEL that could then measure the ratios in other mixtures of substances.
In the longer run, the method opens the exciting perspective to develop a technique for separating variants – a technique that, if successful, could be of great interest to a number of industries, particularly the development of new pharmaceuticals.
More information: Paper: dx.doi.org/10.1038/nature12150
Journal information: Nature
Provided by Harvard University