Catalyst keeps fruit fresh longer
(Phys.org) —Ripening fruit, vegetables, and flowers release ethylene, which works as a plant hormone. Ethylene accelerates ripening, so other unripened fruit also begins to ripen—fruit and vegetables quickly spoil and flowers wilt. In the journal Angewandte Chemie, Japanese researchers have now introduced a new catalytic system for the fast and complete degradation of ethylene. This system could keep the air in warehouses ethylene-free, keeping perishable products fresh longer.
Ethylene is not just a feedstock for the chemical industry; it also acts as a plant hormone, regulating many physiological processes, such as the ripening of fruits and the blooming and wilting of flowers. A familiar example of this is bananas left in a plastic bag, which ripen much faster than those left out. This type of acceleration of ripening even happens in a refrigerator at temperatures around 0 °C.
It is thus very important for wholesalers to remove traces of ethylene from warehouses and cold-storage facilities where fruit, vegetables, and flowers are stored. Previous biotechnological removal methods are expensive, complex, or ineffective. The search for a suitable catalyst for the oxidation of ethylene has also not been very successful. The stumbling block has been the low temperature at which the process must work.
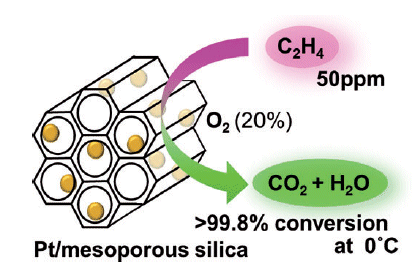
Atsushi Fukuoka and his co-workers at Hokkaido University tested different metals in combination with a variety of support materials to develop an effective catalyst. They met with success: Platinum nanoparticles on a support made of special mesoporous silicon dioxide (MCM-41) demonstrated very high activity in the oxidation of ethylene at 0 to 20 °C. At an ethylene concentration of 50 ppm, over 99.8 % conversion was obtained at 0 °C, a previously unattained level that remains steady over longer periods and after multiple uses.
The catalyst is made by stirring the support with an aqueous solution of a platinum salt for 18 hours. The support is then dried and heated first under oxygen and then under hydrogen. After this process, the large pores of the silicon dioxide material contain platinum particles with a size of about 2.4 nm. This particle size, as well as the effect of the silica, seem to be particularly favorable for the reaction.
It is proposed that ethylene (C2H4) and oxygen initially react rapidly on this catalyst to form formaldehyde (HCHO), which is adsorbed onto the platinum and then primarily degraded to carbon monoxide (CO) and hydrogen species that in turn react with oxygen species to make carbon dioxide and water. A small amount of formic acid is formed as a byproduct. The especially high activity of the catalyst results from the facile oxidation of CO to CO2 that occurs at platinum on silicon dioxide supports. The precise details of the reaction mechanism are currently under investigation.
More information: Fukuoka, A. Low-Temperature Oxidation of Ethylene over Platinum Nanoparticles Supported on Mesoporous Silica, Angewandte Chemie International Edition. dx.doi.org/10.1002/anie.201300496
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Angewandte Chemie





















