'Salted' catalysts for chemical energy storage: Basic alkali-metal salts improve catalyst for methanol steam reforming
(Phys.org) —The storage of hydrogen in the form of methanol is a highly promising method for using excess energy produced by wind and solar power plants. However, this technology requires an effective catalyst for regenerating the hydrogen. German scientists have now introduced a new platinum catalyst for this reaction, known as the steam reforming of methanol, in the journal Angewandte Chemie. The secret of their success lies in a special coating made from molten basic alkali metal salts.
A central problem of renewable energy technology lies in the great variation of energy generated. Unfortunately, wind- and solar-based energy production is not yet possible on a large scale. One possible solution may be offered by methanol-based hydrogen storage. Excess electricity can be used to electrolyze water. The resulting hydrogen can then be reacted with carbon dioxide to make methanol and water, thus allowing it to be stored as a liquid. The hydrogen can be released from the methanol at a later time to power a fuel cell.
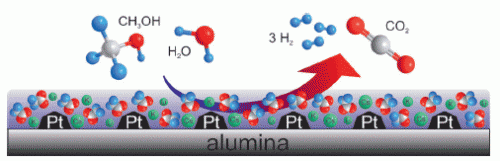
This regeneration of hydrogen is achieved through the steam reforming of methanol, which is essentially a reversal of the methanol forming reaction. In this reaction it is necessary to avoid the formation of carbon monoxide because even the smallest traces of CO would poison the catalysts used in fuel cells. Better catalysts are desperately needed to allow the reforming reaction to work effectively and selectively under decentralized conditions in smaller reactors at the lowest possible temperatures.
A team headed by Peter Wasserscheid and Jörg Libuda at the University of Erlangen-Nürnberg has now developed such an improved catalyst. It uses platinum nanoparticles deposited onto an aluminum oxide support. Most importantly, the surface is coated with a thin film of basic salts, namely a mixture of lithium, potassium, and cesium acetate. Liquid salts have very low vapor pressures so that even under the conditions used for a continuous reaction in the gas phase, they remain on the surface of the catalyst.
The salt coating is so effective because the solubility of the resulting hydrogen in these salts is very low, so that it is rapidly removed from the reaction zone. In addition, the salt is hygroscopic, meaning that it attracts water, and therefore makes the water, which is required for the reaction, readily available at the active sites on the catalyst. The alkali ions also cause the reactants to bind more strongly, while the basic properties of the salt increase the selectivity for CO2.
The coated catalyst has a significantly higher catalytic activity than the uncoated material, and a very significant increase in selectivity toward carbon dioxide to over 99 percent.
More information: Wasserscheid, P. et al. Enhanced Activity and Selectivity in Catalytic Methanol Steam Reforming by Basic Alkali-Metal Salt Coatings, Angewandte Chemie International Edition 2013, 52, No. 19, 5028–5032. dx.doi.org/10.1002/anie.201209758
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Angewandte Chemie