Immune protein C4BP potentially suitable as transporter for drugs
The protein C4BP is similar to a spider in its spatial form with eight "arms". The structure of the "spider body" has recently been described in detail by researchers from the Helmholtz Centre for Infection Research (HZI) in Braunschweig and the Technische Universität Darmstadt. This leads the scientists to unconventional ideas – the protein is possibly suitable as a scaffold for the transport of active pharmaceutical substances, particularly biomolecules. The researchers are publishing their results in the current edition of the international journal Journal of Molecular Biology.
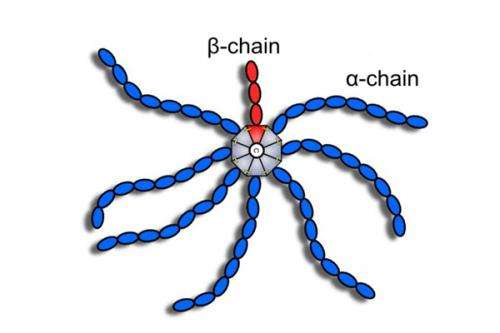
The so-called complement system is a part of the innate immune defence within the human body: more than sixty different proteins form one of the first countermeasures against invading pathogens. One of them is the C4b binding protein known as C4BP. It is involved in the immune defence against bacteria in the blood. How precisely such protein substance carries out its function or how it interacts with other molecules – this can only be predicted by scientists once they have identified the spatial structure of the molecule. Structural biologists therefore examine the substance in its purest form with x-ray machines and are able to reconstruct the spatial design in a computer. Regarding the case of the recently-described C4BP, they found out that it has eight "arms" and thus resembles a spider to a certain degree. Seven of the "arms" are identical as "alpha chains", while the eighth, a "beta chain" is different from the others. The spider body that holds these side chains together is called the oligomerisation domain. Its structure was of special interest to researchers, since it determines the spatial alignment of the "arms".
The newly-described structure allows two possible variants. "However, there is one of these two possibilities that is more feasible because it is much more stable", says Thomas Hofmeyer, PhD student at the Institute for Organic Chemistry and Biochemistry of TU Darmstadt and first author for the publication. And the C4BP is quite stable, as explained by the other first author Dr. Stefan Schmelz from the Department of Molecular Structural Biology of HZI: "Even boiling is not able to break down its form." Usually, human proteins remain stable up to about 40°C. Higher temperatures are of course not found in the body, but the stability of C4BP has a completely different purpose: "As is the case with all components of the complement system, the C4b binding protein is present in blood plasma. The proteins are exposed to enormous shear forces in the blood stream", explains Dr. Andrea Scrima, head of the junior research group "Structural Biology of Autophagy" at HZI. Therefore, the protein needs a high stability in order to be able to withstand these forces.
The researchers now would like to make use of the spatial structure. Their discoveries have facilitated biochemical synthesis of the molecule. In the context of replication within a test tube, the researchers can undertake alterations in a targeted way: "Instead of the seven alpha chains, we could implement other biomolecules", claims Prof. Harald Kolmar, director of the work group Applied Biochemistry at the Institute for Organic Chemistry and Biochemistry at the Technische Universität Darmstadt. "We can use the oligomerisation domain as a framework, in order to decorate it with drug molecules." These could be vaccines, for example. Seven with one stroke, by means of the seven-fold binding capability. Bundled in this manner, more active ingredient could make its way to its target. The dosage could be reduced but the immune system would still be considerably stimulated. "It is thereby possible in the future that bottlenecks, limiting the supply of vaccine, could be avoided and side effects reduced", says Kolmar.
More information: Thomas Hofmeyer, Stefan Schmelz, Matteo T. Degiacomi, Matteo Dal Peraro, Matin Daneschdar, Andrea Scrima, Joop van den Heuvel, Dirk W. Heinz, Harald Kolmar, Arranged Sevenfold: Structural Insights into the C-Terminal Oligomerization Domain of Human C4b-Binding Protein, Journal of Molecular Biology, 2013, DOI: 10.106/j.jmb.2012.12.017
Journal information: Journal of Molecular Biology
Provided by Helmholtz Association of German Research Centres