A new method for measuring the viscosity of nanoparticles
For the first time, scientists measured the chemical diffusivity and viscosity of atmospheric organic particles, thanks to a new approach from scientists at Pacific Northwest National Laboratory, University of Washington, and Imre Consulting. The team doped atmospherically important organic nanoparticles, known as secondary organic aerosols (SOAs), with tracer molecules and measured their diffusion rate as they slowly worked their way out of the particles. Knowing the diffusion rate, the scientists calculated the particle's viscosity.
"Over the past two years, we have shown that long-standing assumptions about the most fundamental properties of SOA particles—phase and volatility—are wrong. Here, for the first time, we quantify chemical diffusivity in SOA particles and show that SOA viscosity is larger—a million times higher than assumed," said lead author Dr. Alla Zelenyuk, physical chemist at Pacific Northwest National Laboratory.
Convenient, but unsubstantiated, assumptions have haunted atmospheric scientists studying SOAs for years, making it impossible to model how the particles affect climate and human health. With the development of new approaches and precise characterization instruments, scientists have disproved the common assumption. With this current study, Zelenyuk and her colleagues have given atmospheric modelers and others data that are invaluable for accurately portraying the particles and their effects in different scenarios, such as new regulations.
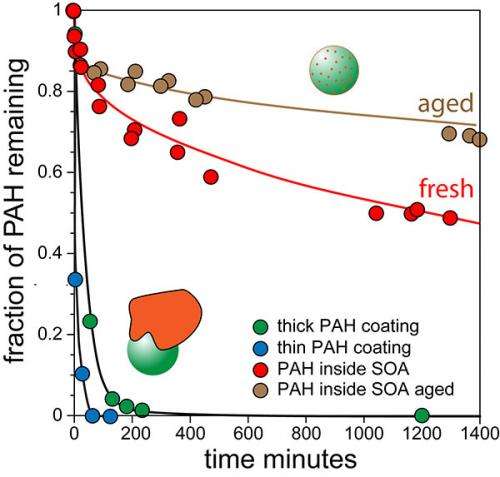
In this first-of-its-kind study, the team began by creating 100-nanometer pyrene-doped SOA particles. They generated the particles via the reaction of ozone with alpha-pinene, the chemical that gives pine trees their smell, in the presence of pyrene vapors. These particles contain a trace amount of hydrophobic or water-fearing pyrene molecules that diffuse through the particles. The team measured the rate with which pyrene molecules diffuse through the particles and evaporate when they reach the surface, from which the team calculated the viscosity of the particles.
"We showed that the particles have viscosity similar to that of tars," said Zelenyuk. "Moreover, with time, they harden, becoming more viscous by a factor of 3 in a day."
The team tested their new method using a host of characterization data. Using approaches that let them characterize multiple dimensions of these particles, such as the temporal evolution of particle size, shape and composition using single particle mass spectrometer, SPLAT II, at EMSL, the team showed that the viscosity measurements matched all other observations.
In addition to answering key questions about the properties of the atmospherically important SOAs, the team demonstrated the effectiveness of their new methodology. "We showed that it is possible to create doped nanoparticles and measure very high viscosity on the time scale of hours to days, instead of years," said Zelenyuk.
The team is working to determine how SOA viscosity and diffusion rates change with temperature and humidity. They are also working with scientists who are developing three-dimensional regional models to see what happens when new findings about SOA are added to atmospheric models.
In addition, the team is working on applying their new method of measuring diffusion and viscosity to other areas. "This method could be extended to other nanoparticle systems that are unrelated to atmospheric particles," said Zelenyuk. "It lets you measure diffusion on the nanoscale at a reasonable time scale."
More information: Imre, A., et al. 2013. Experimental Determination of Chemical Diffusion within Secondary Organic Aerosol Particles. Physical Chemistry Chemical Physics 15:2983-2991. DOI: 10.1039/C2CP44013J
Journal information: Physical Chemistry Chemical Physics
Provided by Pacific Northwest National Laboratory




















