Defects in metal–organic frameworks induce low-temperature ferromagnetism, could yield novel materials for industry
Highly porous materials known as metal–organic frameworks (MOFs) are showing promise as catalysts and drug-delivery vehicles. Some scientific sleuthing by A*STAR researchers could now help industry to exploit the magnetic properties of MOFs for applications such as biomedical sensors.
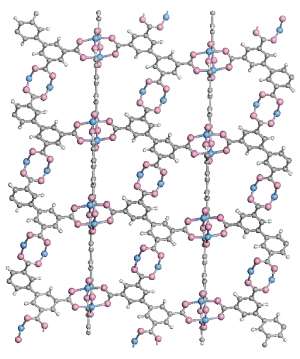
The structure of MOFs resembles atomic scaffolding: clusters of atoms containing metal ions are linked together in a three-dimensional lattice by carbon-based aromatic molecules (see image). One particular MOF—known as HKUST-1—has attracted attention because it was unexpectedly found to be ferromagnetic, albeit at the low temperature of -268.45 °C or less.
Each metal cluster in the material contains a pair of copper ions held together by four carboxylate groups, which contain carbon and oxygen atoms. Each metal ion carries an unpaired electron, which acts like a tiny bar magnet. The magnetic fields of the two unpaired electrons in a cluster would normally oppose each other—the 'north' of one electron lining up with the 'south' of its neighbor—negating any overall magnetism. Even if one copper cluster became magnetic, it would have to align with many other clusters throughout the material to produce ferromagnetism. Yet the organic linker molecules hold the clusters too far apart for the clusters to influence each other directly.
An international team led by Shuo-Wang Yang of the A*STAR Institute of High Performance Computing in Singapore and Lei Shen of the National University of Singapore has now solved the mysterious origin of the ferromagnetism. By modeling the behaviors of ions and electrons in a series of MOFs, the team showed that if a copper ion is absent from a cluster, its carboxylate group will carry an unpaired, magnetic electron instead. Its magnetic field affects itinerant electrons in the MOF's organic linkers, which in turn affect any unpaired electrons in the next copper cluster. If the magnetic message reaches enough clusters, the material as a whole becomes ferromagnetic.
The researchers made a range of MOFs containing the twin copper motif, and found that around 0.57% of the metal ions were indeed missing from the structure—enough to generate ferromagnetism at low temperatures, they calculated. Such copper vacancies are "inevitable, especially for large organic–metal complex systems such as MOFs," the researchers note.
Yang and co-workers also predicted that a MOF containing a non-aromatic organic linker that blocks the magnetic coupling between two adjacent clusters, and confirmed by experiments that it was not ferromagnetic. The researchers now hope to create new ferromagnetic materials by designing MOFs with deliberate metal-ion vacancies.
More information: Chui, S. et al. A chemically functionalizable nanoporous material. Science 283, 1148–1150 (1999). www.ncbi.nlm.nih.gov/pubmed/10024237
Shen, L. et al. Origin of long-range ferromagnetic ordering in metal–organic frameworks with antiferromagnetic dimeric-Cu(II) building units. Journal of the American Chemical Society 134, 17286–17290 (2012). pubs.acs.org/doi/abs/10.1021/ja3077654
Journal information: Science , Journal of the American Chemical Society