New model facilitates predictions about how nanoparticles form, gives clues about how process can be controlled
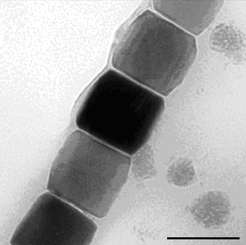
(Phys.org)—Nanoparticles are versatile harbingers of hope: They can serve as active medical agents or contrast media just as well as electronic storage media or reinforcement for structural materials. Researchers from the Max Planck Institute of Colloids and Interfaces in Potsdam-Golm and from the Eindoven University of Technology in the Netherlands made a fundamental contribution to make such nanoparticles usable for these various applications.
While studying magnetite nanoparticles, they developed a model of how crystalline particles of a material form depending on their physical properties. Magnetite nanoparticles are used by some bacteria to orient themselves along the Earth's magnetic field lines. Understanding how they grow could be helpful in generating nanoparticles with the desired properties.
In many respects, material design resembles raising children: many properties are predetermined from nature, others are acquired during education or learning – but the important aspect happens right at the start. A team headed by Damien Faivre, leader of a research group at the Max Planck Institute of Colloids and Interfaces, has looked into the nursery of magnetite nanoparticles.
Magnetite particles that arrange themselves into fine needles serve as a compass for some marine bacteria when they orient themselves along the magnetic field of the Earth in their search for the best living conditions. However, synthetic magnetite particles are also used in inks, magnetic liquids, and medical contrast agent, but also as memory elements in data storage media. With the help of their observations of magnetite nanoparticles, the researchers in Potsdam have expanded the established theory of how crystals of a material form from solution.
The classical model cannot explain the formation of many crystals
In a supersaturated solution, several atoms and molecules agglomerate spontaneously, i.e. more or less randomly, into a seed that then grows further. According to the classical representation of crystal growth, the seed captures atoms or molecules from solution. At that point, either a perfectly ordered crystal can form directly or an amorphous, and thus disordered, conglomeration forms first, which then rearranges itself into a crystal.
Which of the two pathways the crystal evolves in, depends on which one exhibits the lower energy level – the crystalline phase or the disordered one. The determining properties here are the surface energies of the crystalline and disordered variants, as well as the amounts of energy that are released when atoms or molecules bind to the one or the other form. A high surface energy drives the energy expenditure for the given phase's growth much higher, while a large energy yield from the evolving bonds lowers it.
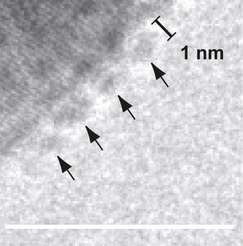
"Over the years there have been increasing indications that numerous minerals do not grow according to this model", says Damien Faivre. "They apparently take up neither single atoms nor molecules during their formation, but instead capture primary particles or clusters up to a few nanometres in size that only form temporarily." That is more or less what happens when crystals of calcium carbonate and calcium phosphate form that harden bones or mollusc shells. Faivre and his team have now established that magnetite nanoparticles also grow by absorbing small primary particles only two nanometres in size. The researchers observed this with a transmission electron microscope operated at a temperature well below zero that thus depicts especially small structures.
The stability of the primary particles becomes the decisive factor
"Using the classical model, it is impossible to determine whether larger nanocrystals form from the small nanoparticles directly or whether a disordered phase forms first", says Damien Faivre. However, if you want to grow nanoparticles, you must be able to answer this question. So he and his colleagues developed a new model (that takes into account the primary particles).
In the new model, the stability of the nanoparticles becomes an important factor - so important that it can even reverse the prediction of the classical model. "The more stable the primary particles are, the more likely a crystalline structure forms directly", explains Faivre. "In many cases, when a disordered phase should form under the classical model, our model results in a crystal forming directly." This is exactly the case with magnetite.
Investigating the primary particles is the next step
Whether crystals grow according to the classical model or the one proposed by Damien Faivres' team depends on whether atoms and molecules or the miniscule primary particles are involved. "You either know this through observations, as in our case, or you anticipate it with the help of the material's physical properties", explains Faivre.
However, the researchers still have numerous unresolved questions to answer in order to move from these insights about the nanoparticle nursery to an instruction manual for directing their growth. "In the next step, we will investigate the primary particles and their properties more precisely", says Damien Faivre. If the researchers can control the stability of the particles assimilated by a growing nanoparticle, they may also have a means of influencing the properties of the nanoparticle. This is hardly different than with young, growing children: what they become depends on how they are fed.
More information: Baumgartner, J. et al. Nucleation and growth of magnetite from solution, Nature Materials, published online February 3, 2013; DOI: 10.1038/NMAT3558
Journal information: Nature Materials
Provided by Max Planck Society