Researchers explore more efficient carbon dioxide to methanol model
Researchers from The University of Texas at Arlington are pioneering a new method for using carbon dioxide, or CO2, to make liquid methanol fuel by using copper oxide nanowires and sunlight.
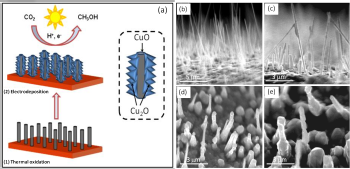
The process is safer, simpler and less expensive than previous methods to convert the greenhouse gas associated with climate change to a useful product, said Krishnan Rajeshwar, interim associate vice president for research at UT Arlington and one of the authors of a paper recently published in the journal Chemical Communications. Researchers began by coating the walls of copper oxide, CuO, nanorods with crystallites made from another form of copper oxide, Cu2O. In the lab, they submerged those rods in a water-based solution rich in CO2. Irradiating the combination with simulated sunlight created a photoelectrochemical reduction of the CO2 and that produced methanol.
In contrast, current methods require the use of a co-catalyst and must be conducted at high operating pressures and temperatures. Many also use toxic elements, such as cadmium, or rare elements, such as tellurium, Rajeshwar said.
"As long as we are using fossil fuels, we'll have the question of what to do with the carbon dioxide," said Rajeshwar, a distinguished professor of chemistry and biochemistry and co-founder of the Center for Renewable Energy, Science & Technology, CREST, at UT Arlington. "An attractive option would be to convert greenhouse gases to liquid fuel. That's the value-added option."
Co-authors on the recently published paper, "Efficient solar photoelectrosynthesis of methanol from carbon dioxide using hybrid CuO-Cu2O semiconductor nanorod arrays," are Ghazaleh Ghadimkhani, Norma Tacconi, Wilaiwan Chanmanee and Csaba Janaky, all of the UT Arlington College of Science's Department of Chemistry and Biochemistry and CREST. Janaky also has a permanent appointment at the University of Szeged in Hungary.
Rajeshwar said he hopes that others will build on the research involving copper oxide nanotubes, CO2 and sunlight.
"Addressing tomorrow's energy needs and finding ways to stem the harmful effect of greenhouse gases are areas where UT Arlington scientists can connect their work to real-world problems," said Carolyn Cason, vice president for research at the University. "We hope solutions in the lab are only the beginning."
In addition to the journal, the new work also was featured in a recent edition of Chemical and Engineering News. That piece noted that the experiments generated methanol with 95 percent electrochemical efficiency and avoided the excess energy input, also known as overpotential, of other methods.
Tacconi, a recently retired research associate professor at UT Arlington, said the two types of copper oxide were selected because both are photo active and they have complementary solar light absorption. "And what could be better in Texas than to use the sunlight for methanol generation from carbon dioxide?"
Other than fuel, methanol is used in a wide variety of chemical processes, including the manufacturing of plastics, adhesives and solvents as well as wastewater treatment. In the United States, there are 18 methanol production plants with a cumulative annual capacity of more than 2.6 billion gallons, according to the paper.
Journal information: Chemical Communications
Provided by University of Texas at Arlington