Evolutionary biologists urged to adapt their research methods
To truly understand the mechanisms of natural selection, evolutionary biologists need to shift their focus from present-day molecules to synthesized, ancestral ones, says Shozo Yokoyama, a biologist at Emory University.
Yokoyama will present evidence for why evolutionary biology needs to make this shift on Friday, February 15, during the American Academy of Arts and Sciences (AAAS) annual meeting in Boston.
"This is not just an evolutionary biology problem, it's a science problem," says Yokoyama, a leading expert in the natural selection of color vision. "If you want to understand the mechanisms of an adaptive phenotype, the function of a gene and how that function changes, you have to look back in time. That is the secret. Studying ancestral molecules will give us a better understanding of genes that could be applied to medicine and other areas of science."
For years, positive Darwinian selection has been studied almost exclusively using comparative sequence analysis of present-day molecules, Yokoyama notes. This approach has been fueled by increasingly fast and cheap genome sequencing techniques. But the faster, easier route, he says, is not necessarily the best one if you want to arrive at a true, quantitative result.
"If you only study present-day molecules, you're only getting part of the picture, and that picture is often wrong," he says.
Yokoyama has spent two decades teasing out secrets of the adaptive evolution of vision in fish and other vertebrates.
Five classes of opsin genes encode visual pigments and are responsible for dim-light and color vision. Fish provide clues for how environmental factors can lead to vision changes, since the available light at various ocean depths is well quantified. The common vertebrate ancestor, for example, possessed ultraviolet vision, which is suited to both shallow water and land.
"As the environment of a species sinks deeper in the ocean, or rises closer to the surface and moves to land, bits and pieces of the opsin genes change and vision adapts," Yokoyama says. "I'm interested in exactly how that happens at the molecular level."
Molecular biologists can take DNA from an animal, isolate and clone its opsin genes, then use in vitro assays to construct a specific visual pigment. The pigment can be manipulated by changing the positions of the amino acids, in order to study the regulation of the gene's function.
In 1990, for example, Yokoyama identified the three specific amino acid changes that switch the human red pigment into a green pigment.
A few years later, another group of researchers confirmed Yokoyama's findings, but found that the three changes only worked in one direction. In order to reverse the process, and turn the green pigment back to red, it took seven changes.
"They discovered this weird quirk that didn't make sense," Yokoyama says. "Why wouldn't it take the same number of changes to go in either direction? That question was interesting to me."
He spent 10 years researching and pondering the question before he realized the key problem: The experiments were conducted on present-day molecules.
When the earliest mammalian ancestors appeared 100 million years ago, they had only the red pigment. Around 30 million years ago, the gene for the red pigment duplicated itself in some primates. One of these duplicated red pigments then acquired sensitivity to the color green, turning into a green pigment.
"At the point that the three changes in amino acids occurred in this pigment, other mutations were happening as well," Yokoyama says. "You have to understand the original interactions of all of the amino acids in the pigment, which means you have to look at the ancestral molecules. That's the trick."
In other words, just as changes in an animal's external environment drive natural selection, so do changes in the animal's molecular environment.
Statistical analysis allows Yokoyama and his collaborators to travel back in time and estimate the sequences for ancestral molecules. "It's a lot of work," he says. "We don't have a clear picture of every intermediate species. We have to do a step-by-step retracing, screening for noise in the results at each step, before we can construct a reliable evolutionary tree."
In 2008, Yokoyama led an effort to construct the most extensive evolutionary tree for dim-light vision, including animals from eels to humans. At key branches of the tree, Yokoyama's lab engineered ancestral gene functions, in order to connect changes in the living environment to the molecular changes.
The lengthy process of synthesizing ancestral proteins and pigments and conducting experiments on them combines microbiology with painstaking techniques of theoretical computation, biophysics, quantum chemistry and genetic engineering.
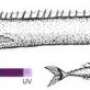
This multi-dimensional approach allowed Yokoyama's lab in 2009 to identify the scabbardfish as the first fish known to have switched from ultraviolet vision to violet vision. And Yokoyama pinpointed exactly how the scabbardfish made the switch, by deleting an amino acid molecule at site 86 in the chain of amino acids in the opsin gene.
"Experimenting on ancestral molecules is the key to getting a correct answer to problems of natural selection, but there are very few examples of that being done in evolutionary biology," Yokoyama says.
Provided by Emory University