Researchers solve the 3-D crystal structure of one of the most important human proteins
(Phys.org)—A research team at Weill Cornell Medical College has solved the 3D crystal structure of a member protein in one of the most important classes of human proteins—the G protein-coupled receptors (GPCRs). These types of proteins latch on to and transmit chemical signals from outside the cell to the inside, and half of all drugs on the market today work by ether inhibiting or activating GPCRs.
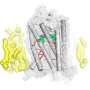
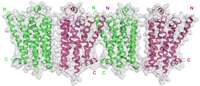
The discovery, detailed in Nature Structural & Molecular Biology, shows the crystal structure of a GPCR—the beta 1-adrenergic receptor—that does not have a chemical signal or a "ligand" bound to it. The researchers say the finding will likely offer a major boost to drug development because designers can use information gleaned from the crystal structure to learn how to build new, more effective drugs.
"Now, by understanding the native structure of these receptors—which are likely very similar to each other—drug designers may be able to create therapies that are exquisitely targeted. That can produce better therapeutic results for patients while minimizing side effects," says Dr. Xin-Yun Huang, a professor of physiology and biophysics at Weill Cornell Medical College.
It was notoriously difficult to crystallize this ligand-free membrane receptor, which explains why no one has been able to solve a GPCR structure without ligands before, Dr. Huang adds. One scientist who managed to solve the structures of several GPCRs bound to their ligands, and also capture the structure of a GPCR bound to the G protein it usually activates on the inside of a cell, was awarded the 2012 Nobel Prize in Chemistry.
The atomic view of the unliganded GPCR has already offered some surprises to Dr. Huang and his Weill Cornell research team.
"No one knew what a GPCR at its starting, basic unliganded state looked like—or what to expect," he says. "We found that the ligand-free beta 1-adrenergic receptors form oligomers. Identification of this structure type is important because it may provide the structural basis for the communication among receptors, and between receptors and G proteins."
Mysterious workings of GPCR targeted drugs
GPCRs are the largest group of cell surface receptors involved in signal transduction. They transmit signals from an enormous array of stimuli, everything from photons (light) to odorants, hormones, growth factors and neurotransmitters, says Dr. Huang, whose research has long focused on the GPCRs and the G proteins they activate inside a cell. The G proteins amplify and transfer the signal from GPCRs to produce a biochemical response.
This GPCR-G protein signaling system plays critical roles in various physiological processes such as cardiovascular and neurological functions, and in human diseases such as cancer. Drugs are designed to bind on the GPCRs and activate them, reduce their activity or turn their activity off. For example, the beta 1-adrenergic receptor on the outside of heart cells that Dr. Huang and his team crystallized is the target of beta-blocker drugs that slow down heart beat.
Many drugs that target GPCRs have been discovered by blindly screening large libraries of drug-like small molecules. Recently, crystal structures of GPCRs bound to ligands have helped researchers design new drugs. Drugs that latch on to the same binding site on a GPCR may work to either activate or inhibit transmission of a signal.
"It may be possible to compare the atomic structures of the ligand-free receptor in its starting state, when it is bound by a ligand that activates it and when it is bound by a ligand that inhibits it. The small differences may offer us clues to develop agents that elicit the reaction we want," says Dr. Huang.
Dr. Huang is now working to solve the 3D structure of the beta 1-adrenergic receptor linked to its partner G protein. "This may also provide a new template for designing new and more effective medications to control heart function," he says.
Journal information: Nature Structural & Molecular Biology
Provided by Weill Cornell Medical College