Surfactant chemical enables researchers to extract significant oil deposits, leaves positive environmental footprint

Chemicals found in common household items, like toothpaste and soap, are proving to be the right formula to safely extract up to 70 percent of the oil still embedded in high-salt oil reservoirs in the United States.
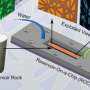
With controversy surrounding advance recovery methods like fracking, a team from the University of Oklahoma Institute for Applied Surfactant Research – Jeff Harwell, Ben Shiau and Bruce Roberts – has formulated an environmentally sound compound that increases oil flow in previously pumped reservoirs. By using a surfactant that decreases the surface tension, oil is released from the rock so it can move with the injected water and be pushed to the production wells safely.
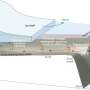
Secondary recovery methods, such as water flooding and hydraulic fracturing, are used to recover oil left behind by previously pumped reservoirs. The methods drive trapped oil toward the drill hole, but when the injected water reaches the production wells, most of the oil remains trapped in the rock, much like a sponge traps water.
"Our surfactants replace the crude oil within the rock with harmless compounds like brine that maintain the integrity of the rock formation," Harwell said. "The ingredients we use are in things that people use every day to bathe, brush their teeth and wash their car. The chemicals we inject are not near the threat or hazard level of the compounds in the oil we are removing from the reservoir."
The OU teams' surfactant increases flooding efficiency even at concentrated levels of salinity. The OU team is the only group in the United States that focuses its work at such high salt levels.
"Most research focuses on salinity around a 3 to 5 percent threshold," Harwell said. "We recently successfully formulated a surfactant system for up to 26 percent salinity."
The OU research currently is focused on oil in Oklahoma's Pennsylvanian aged sands that span much of the state and typically contain high concentrations of salt, ranging from 15 to 25 percent.
"That means there is a huge area with a tremendous amount of oil trapped in these formations," Harwell said. "The Oklahoma Geological Survey estimates there were 84 billion barrels of original oil in these reservoirs and we have only extracted about 15 billion."
The research team has successfully tested its surfactant formulations in several single-well operations and is now moving to small, multiple-well testing. If successful, the surfactant would enable small oil producers to recover more oil efficiently and cost effectively, while leaving the formations environmentally sound.
Provided by University of Oklahoma