Study quantifies the size of holes antibacterials create in cell walls to kill bacteria
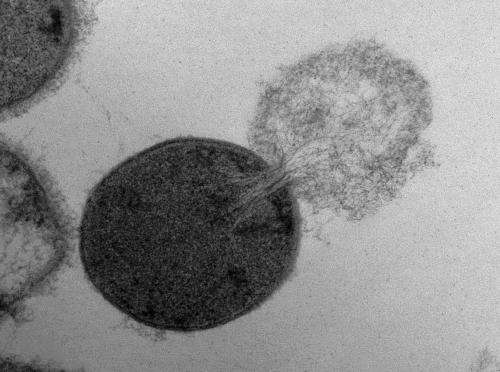
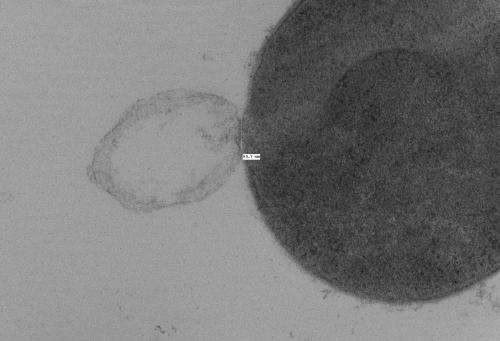
Researchers recently created a biophysical model of the response of a Gram-positive bacterium to the formation of a hole in its cell wall, then used experimental measurements to validate the theory, which predicted that a hole in the bacteria cell wall larger than 15 to 24 nanometers in diameter would cause the cell to lyse, or burst.
The rise of antibiotic-resistant bacteria has initiated a quest for alternatives to conventional antibiotics. One potential alternative is PlyC, a potent enzyme that kills the bacteria that causes strep throat and streptococcal toxic shock syndrome. PlyC operates by locking onto the surface of a bacteria cell and chewing a hole in the cell wall large enough for the bacteria's inner membrane to protrude from the cell, ultimately causing the cell to burst and die.
Research has shown that alternative antimicrobials such as PlyC can effectively kill bacteria. However, fundamental questions remain about how bacteria respond to the holes that these therapeutics make in their cell wall and what size holes bacteria can withstand before breaking apart. Answering those questions could improve the effectiveness of current antibacterial drugs and initiate the development of new ones.
Researchers at the Georgia Institute of Technology and the University of Maryland recently conducted a study to try to answer those questions. The researchers created a biophysical model of the response of a Gram-positive bacterium to the formation of a hole in its cell wall. Then they used experimental measurements to validate the theory, which predicted that a hole in the bacteria cell wall larger than 15 to 24 nanometers in diameter would cause the cell to lyse, or burst. These small holes are approximately one-hundredth the diameter of a typical bacterial cell.
"Our model correctly predicted that the membrane and cell contents of Gram-positive bacteria cells explode out of holes in cell walls that exceed a few dozen nanometers. This critical hole size, validated by experiments, is much larger than the holes Gram-positive bacteria use to transport molecules necessary for their survival, which have been estimated to be less than 7 nanometers in diameter," said Joshua Weitz, an associate professor in the School of Biology at Georgia Tech. Weitz also holds an adjunct appointment in the School of Physics at Georgia Tech.
The study was published online on Jan. 9, 2013 in the Journal of the Royal Society Interface. The work was supported by the James S. McDonnell Foundation and the Burroughs Wellcome Fund.
Common Gram-positive bacteria that infect humans include Streptococcus, which causes strep throat; Staphylococcus, which causes impetigo; and Clostridium, which causes botulism and tetanus. Gram-negative bacteria include Escherichia, which causes urinary tract infections; Vibrio, which causes cholera; and Neisseria, which causes gonorrhea.
Gram-positive bacteria differ from Gram-negative bacteria in the structure of their cell walls. The cell wall constitutes the outer layer of Gram-positive bacteria, whereas the cell wall lies between the inner and outer membrane of Gram-negative bacteria and is therefore protected from direct exposure to the environment.
Georgia Tech biology graduate student Gabriel Mitchell, Georgia Tech physics professor Kurt Wiesenfeld and Weitz developed a biophysical theory of the response of a Gram-positive bacterium to the formation of a hole in its cell wall. The model detailed the effect of pressure, bending and stretching forces on the changing configuration of the cell membrane due to a hole. The force associated with bending and stretching pulls the membrane inward, while the pressure from the inside of the cell pushes the membrane outward through the hole.
"We found that bending forces act to keep the membrane together and push it back inside, but a sufficiently large hole enables the bending forces to be overpowered by the internal pressure forces and the membrane begins to escape out and the cell contents follow," said Weitz.
The balance between the bending and pressure forces led to the model prediction that holes 15 to 24 nanometers in diameter or larger would cause a bacteria cell to burst. To test the theory, Daniel Nelson, an assistant professor at the University of Maryland, used transmission electron microscopy images to measure the size of holes created in lysed Streptococcus pyogenes bacteria cells following PlyC exposure.
Nelson found holes in the lysed bacteria cells that ranged in diameter from 22 to 180 nanometers, with a mean diameter of 68 nanometers. These experimental measurements agreed with the researchers' theoretical prediction of critical hole sizes that cause bacterial cell death.
According to the researchers, their theoretical model is the first to consider the effects of cell wall thickness on lysis.
"Because lysis events occur most often at thinner points in the cell wall, cell wall thickness may play a role in suppressing lysis by serving as a buffer against the formation of large holes," said Mitchell.
The combination of theory and experiments used in this study provided insights into the effect of defects on a cell's viability and the mechanisms used by enzymes to disrupt homeostasis and cause bacteria cell death. To further understand the mechanisms behind enzyme-induced lysis, the researchers plan to measure membrane dynamics as a function of hole geometry in the future.
More information: Mitchell GJ, Wiesenfeld K, Nelson DC, Weitz JS, "Critical cell wall hole size for lysis in Gram-positive bacteria," J R Soc Interface 20120892 (2013): dx.doi.org/10.1098/rsif.2012.0892
Journal information: Journal of the Royal Society Interface
Provided by Georgia Institute of Technology