New options for transparent contact electrodes
Found in flat screens, solar modules, or in new organic light-emitting diode (LED) displays, transparent electrodes have become ubiquitous. Typically, they consist of metal oxides like In2O3, SnO2, ZnO and TiO2.
But since raw materials like indium are becoming more and more costly, researchers have begun to look elsewhere for alternatives. A new review article by HZB scientist Dr. Klaus Ellmer, published in the renowned scientific journal Nature Photonics, is hoping to shed light on the different advantages and disadvantages of established and new materials for use in these kinds of contact electrodes.
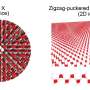
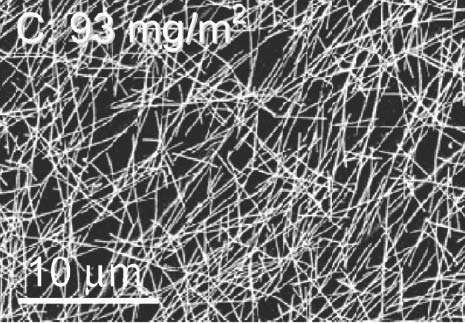
Metallic (Ag or Cu) or carbon based nanostructures exhibit many interesting properties that could potentially be exploited pending further research. Even graphene, a modified form of carbon, could turn out to be a suitable transparent electrode, since it is both transparent and highly conductive. These properties depend, to a large extent, on the material's composition: graphene, which consists of a single layer of carbon atoms arranged into a hexagonal "honeycomb" grid, is two-dimensional, and, within these dimensions, electrons can freely move about.
According to Ellmer, "these new kinds of materials could be combined with more conventional solutions or find their way into entirely new areas of application." For this to become a reality, researchers have yet to come up with solutions to nanostructure problems like short circuits and continue to illuminate the relevant transport mechanisms. It would also be interesting to determine whether these two-dimensional "electron gases" also form in materials other than graphene. Success ultimately depends on whether or not the new materials prove stable in the long run in their practical application and whether or not they can be produced relatively inexpensively.
More information: Ellmer is sole author of an extensive review article published in Nature Photonics online on 30. November 2012, doi: 10.1038/nphoton.2012.282
Journal information: ACS Nano , Nature Photonics
Provided by Helmholtz Association of German Research Centres