Molecular twist helps regulate the cellular message to make histone proteins
Histone proteins are the proteins that package DNA into chromosomes. Every time the cell replicates its DNA it must make large amounts of newly made histones to organize DNA within the nucleus.
An imbalance in the production of DNA and histones is usually lethal for the cell, which is why the levels of the messenger RNA (mRNA) encoding the histone proteins must be tightly controlled to ensure the proper amounts of histones (not too many and not too few) are made.
In a collaborative effort published online in the January 18, 2013 issue of the journal Science, researchers at the University of North Carolina and Columbia University show for the first time how two key proteins in messenger RNA communicate via a molecular twist to help maintain the balance of histones to DNA.
"This is one of the safeguards that our cells have evolved and it is part of the normal progression through cell division – all growing cells have to use this all of the time," said study co-author William F. Marzluff, PhD, Kenan Distinguished Professor of biochemistry and biophysics at UNC's School of Medicine.
Every time a cell divides, Marzluff adds, it has to replicate both DNA and histone proteins and then package them together into chromosomes. "That way, each of the two cells resulting from division has one complete set of genes."
In humans, the 23 chromosomes that house roughly 35,000 genes are made up of both DNA and histone proteins. The DNA for a histone protein is first transcribed into RNA, which then acts as a guide for building a histone protein. Because the RNA relays a message – in this case a blueprint for a histone protein, it is referred to as messenger RNA, or mRNA.
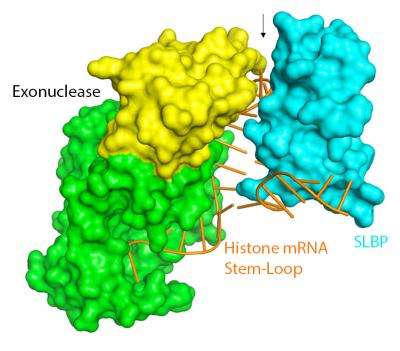
Histone mRNAs differ from all other mRNAs and end in a stem-loop [or hairpin] sequence that is required for proper regulation of histone mRNAs. In this study, the Columbia team of Liang Tong, PhD, Professor of biological sciences and the corresponding author on this project, and graduate student Dazhi Tan used crystallography to reveal the structure of two important proteins near the end of the histone mRNA stem-loop. This molecular complex is required for regulating the levels of the histone mRNA.
One of these proteins, stem-loop binding protein (SLBP) is required for translation of histone mRNA into protein, and the other is an exonuclease, which is required to destroy the mRNA. Both were initially identified at UNC by Marzluff and colleague Zbigniew Dominski, PhD, Professor of biochemistry and biophysics, also a study co-author.
"We knew there was some interaction between SLBP and the exonuclease, so we asked Liang to explain how they bind and communicate," Dominski said. "And the surprising thing was that the proteins do it not by binding to each other but by changing the RNA structure at the site."
"From the science point of view, that was the most dramatic thing," Marzluff said. "The way these proteins help each other is either one can twist the RNA so the other can recognize it easier, and they don't have to touch each other to do that."
This protein complex is a critical regulator of histone synthesis, and is an important component of cell growth, he adds. "Interfering with it could provide a new method for interfering with cancer cell growth."
Journal information: Science
Provided by University of North Carolina Health Care
















