'Green chemistry' using carbon dioxide, low-cost catalysts: New way of producing potent carbon–boron synthetic reagents
Because carbon dioxide (CO2) gas is a freely available resource, there are concerted efforts worldwide to convert this molecule into a chemical feedstock. Zhaomin Hou and colleagues from the RIKEN Advanced Science Institute in Wako have made important progress toward this goal by developing the first protocol for attaching both CO2 and boron atoms to unsaturated carbon–carbon triple bonds. This procedure uses inexpensive organic–copper catalysts to construct valuable 'building blocks' for chemists under mild, one-pot conditions.
The strong double bonds inside CO2 make this molecule particularly inert and hard to use in most chemical reactions. Current tactics have focused on using transition metals to catalyze addition of electron-rich organic 'nucleophiles' to CO2's central carbon atom. This technique has successfully generated simple carboxylic acids. However, production of more complex substances containing non-hydrocarbon atoms has remained mostly out of reach.
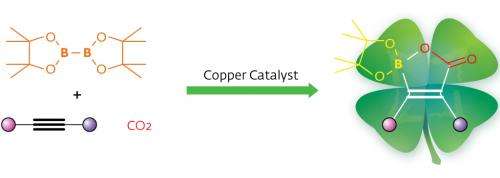
Hou and his team used a groundbreaking approach to help turn CO2 into organoboron reagents—valuable synthetic compounds because of the wide number of transformations possible at carbon–boron bonds. First, they turned alkynes, molecules with carbon–carbon triple bonds, into nucleophiles. Nucleophilic species are highly reactive with many types of chemical groups but they are also difficult to control. To achieve necessary precision, the team used N-heterocyclic carbene (NHC) copper complexes, a hybrid organic/inorganic system with a strong track record of catalyzing CO2 additions.
X-ray experiments revealed that the strategy had paid off: NHC–copper complexes could indeed catalyze the addition of CO2 and diborane molecules to alkynes through a three-step catalytic insertion process. This reaction generates a final product with a unique, previously unknown cyclic structure containing a boron atom, a carbon–carbon double bond and a carboxyl group that the authors termed 'boralactone'.
By tweaking the structure of the NHC–copper catalyst, the researchers were able to apply the technique to a wide range of alkyne-type molecules with no side reactions. Intriguingly, the catalyst delivered the same geometric arrangement—high regio- and stereoselectivity—no matter which substituents were attached to the carbon triple bond. Hou explains that this advantageous behavior occurs because the diborane–catalyst complex always attacks the alkyne bond from a specific direction due to electronic interactions. Furthermore, the cyclic boralactone helps to drive this selectivity.
"Our reaction may serve as an attractive method for the synthesis of multifunctional alkenes, as it uses CO2 and easily available alkynes as building blocks with a relatively cheap copper catalyst," concludes Hou.
More information:
References:
Zhang, L., Cheng, J., Carry, B. & Hou, Z. Catalytic boracarboxylation of alkynes with diborane and carbon dioxide by an N-heterocyclic carbene copper catalyst. Journal of the American Chemical Society 134, 14314–14317 (2012). pubs.acs.org/doi/abs/10.1021/ja3063474
Ohishi, T., Nishiura, M. & Hou, Z. Carboxylation of organoboronic esters catalyzed by N-heterocyclic carbene copper(I) complexes. Angewandte Chemie International Edition 47, 5792–5795 (2008). onlinelibrary.wiley.com/doi/10 … e.200801857/abstract
Journal information: Journal of the American Chemical Society , Angewandte Chemie International Edition
Provided by RIKEN