Cell biologists identify new protein key to asymmetric cell division
Recently biologists at the University of Massachusetts Amherst led by Wei-lih Lee have identified a new molecular player in asymmetric cell division, a regulatory protein named She1 whose role in chromosome- and spindle positioning wasn't known before. Asymmetric cell division is important in the self-renewal of stem cells and because it ensures that daughter cells have different fates and functions.
When a fertilized egg develops in a fruit fly or a human being, the number of asymmetric cell divisions must be precisely balanced by symmetric cell divisions, Lee explains. He has spent years studying the cell's molecular engine called dynein, which in many cases controls how embryos accomplish asymmetric cell division, though exactly how is not completely understood.
Now, Lee and postdoctoral researcher Steven Markus, with undergraduate Katelyn Kalutkiewicz, in experiments supported by the NIH's National Institute of General Medical Sciences, have identified She 1 as the first known regulator of asymmetric cell division that inhibits the dynein engine, but surprisingly also promotes asymmetric division. Their work is described in an early online edition of Current Biology and will appear in the December 4 print edition.
Working with common yeast, Lee explains, "With this study, we've looked deeper than ever before into dynein and its role in asymmetric cell division. This is a highly conserved process that's very important to human development, to tissue differentiation and the self-renewing process of stem cells. Many had hypothesized that dynein influences the outcome of the division by pulling on the mitotic spindle, the intricate machine responsible for separating chromosomes. How dynein knows which direction to pull the spindle had become the holy grail of this research."
When a cell is ready to divide asymmetrically, dynein molecules move to its outer wall in opposite directions by riding along microtubules "tracks" and anchoring in the wall like tent stakes. Until Lee and colleagues' recent discovery of She1's role, biologists believed dynein acted alone to direct the spindle apparatus between them, pulling chromosomes apart like taffy to form two daughter cells. They thought She1 seemed to be involved in dynein distribution, as it was often observed on the microtubule tracks along which dynein draws the spindle apparatus.
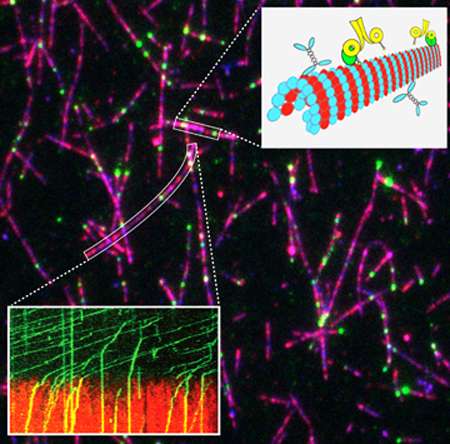
To explore She1's secrets, Lee and his team used Total Internal Reflection Fluorescence (TIRF) microscopy, which allows them to visualize single molecules at high resolution. They can watch the dynein engine as it powers along the microtubule tracks.
"We were curious," Lee notes. "We knew She1 was present but we didn't know why. So we decided to do some biochemistry with it in the microscope. Steven purified the protein and designed experiments to see if it interacted with the motor protein. And it did."
In fact, the UMass Amherst research team found that She1 interacts with dynein only when the motor protein is on the microtubule cable and the motor is moving on the track, never elsewhere. "We observed them colliding on the track, then binding. The new concept is that these microtubules become different," Lee adds. "Discovering that She1 can block dynein's motoring totally changed our thinking about how the spindle is being pulled in the cell."
The researchers were surprised because observing its behavior, one would predict that She1 inhibits asymmetric division by blocking dynein. It turns out that is not true. Instead, She1 actively promotes asymmetric cell division by changing its local underlying microtubules. Tracks containing She1 no longer permit dynein to pass.
It's a subtle difference, Lee acknowledges, but important. He clarifies, "We think the microtubule tracks might be 'licensed,' so to speak, by She1. The idea had been that the engine was always 'on' and pulling. However, now we have identified this new player with the ability to specifically regulate this pulling very locally. If there's high She1 concentration on one side of the spindle, dynein can only pull from the other side, thus specifying the direction of the pulling."
There is no counterpart to She1 found in common yeast yet known in humans, but Lee and colleagues expect a similar protein will be discovered that regulates and directs dynein's pulling in asymmetric cell division.
Journal information: Current Biology
Provided by University of Massachusetts Amherst