Light and air: Sunlight-driven CO2 fixation
(Phys.org)—The increased use of renewable energy sources, particularly sunlight, is highly desirable, as is industrial production that is as CO2-neutral as possible. Both of these wishes could be fulfilled if CO2 could be used as the raw material in a system driven by solar energy. Japanese researchers have now introduced an approach to this type of process in the journal Angewandte Chemie. Their method is based on a principle similar to natural photosynthesis.
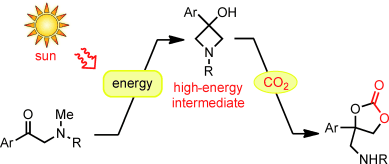
The use of carbon dioxide as a source of carbon may be an attractive option for reducing the consumption of fossil feedstocks and improving the CO2 footprint of chemical products. The biggest obstacle in our way is the high stability of the CO2 molecule. One of the possibilities for jumping this hurdle is to use very high-energy molecules to react with CO2. The photosynthetic process in green plants provides an example of how this could work. This process takes place in two steps: the light reactions and the dark reactions. In the light reactions, the photosynthetic system captures photons and stores their energy in the form of energetic chemical compounds. These are subsequently used to drive the dark reactions that use CO2 as a carbon source to synthesize complex sugar molecules.
Researchers working with Masahiro Murakami at Kyoto University used the same principle to design their process. In this case, the first step is also a reaction driven by light. The action of UV light can convert the starting material, an α-methylamino ketone, to a very energetic molecule. This also works with sunlight, as the researchers found out. An intramolecular rearrangement with ring closure results in a molecule containing a ring made of three carbon atoms and one nitrogen atom. This type of ring is under a great deal of strain and is correspondingly reactive. This "light reaction" was coupled to a "dark reaction": In the subsequent light-independent step, the highly energetic compound captures CO2 in the presence of a base. This forms a cyclic amino-substituted carbonic acid diester that could be useful as an intermediate for chemical syntheses.
The striking thing about this reaction scheme is that the technique is simple. Diffuse sunlight on cloudy days is enough to drive the process. The second step can be carried out in the same reaction vessel through simple addition of the base and heating to 60 °C. The yield is 83 %. In addition, the process is very adaptable because a wide variety of α-methylamino ketones can be used as starting materials.
More information: Murakami, M. Solar-Driven Incorporation of Carbon Dioxide into α-Amino Ketones, Angewandte Chemie International Edition 2012, 51, No. 47, 11750–11752. dx.doi.org/10.1002/anie.201206166
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Angewandte Chemie