Made out of thin air: Fixation of CO2 through iridium catalyzed hydrosilylation
(Phys.org)—Carbon dioxide could be a useful alternative source of carbon for the chemical industry. It is inexpensive, is supplied in abundance by nature, and would help to reduce the consumption of fossil fuels. In addition, it would significantly improve the carbon footprint of fuels and chemical products. The largest barrier to this process is the high stability of the carbon dioxide molecule. In the journal Angewandte Chemie, Spanish researchers have now introduced a new process that traps carbon dioxide in the form of silyl formates, which are silicon-containing formic acid esters.
The hydrogenation of CO2 to formic acid (HCO2H) is an area of CO2 extraction that is being intensively researched. In the chemical industry, formic acid is used as a starting material for many products, with applications including agriculture, food technology, and the leather goods industry. Most interestingly, it could be used as a hydrogen-storage medium for fuel-cell-driven vehicles.
Although a number of catalytic processes for the production of formic acid from CO2 have been developed, none of them have been implemented industrially. The reaction is an equilibrium that significantly favors the reactants. In order to hinder the constantly running reverse reaction, the formic acid must be trapped—in the form of salts, adducts, or derivatives—in order to remove it from the equilibrium.
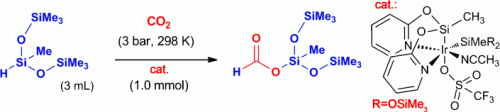
A team led by Francisco J. Fernández-Alvarez and Luis A. Oro at the University of Zaragoza has now developed a new catalyst that allows carbon dioxide to be converted and trapped as a silyl formate. These compounds can be used for the production of silicone polymers and as reactive intermediates in organic syntheses. It is also easily possible to release formic acid from the silyl formate.
The new reaction, which the researchers have been able to carry out on a gram scale, occurs under very mild reaction conditions. It is highly selective and delivers a high turnover, works without a solvent and produces no waste products. The carbon dioxide is reduced by heptamethyltrisiloxane. At the heart of the reaction lies a specially developed iridium catalyst that is formed in situ from an air- and water-stable precursor.
More information: Luis A. Oro, Effective Fixation of CO2 by Iridium-Catalyzed Hydrosilylation. Angewandte Chemie International Edition. dx.doi.org/10.1002/anie.201206165
Journal information: Angewandte Chemie International Edition
Provided by Wiley