Synthetic liver enzyme could result in more effective drugs with fewer side effects
(Phys.org)—Medicines could be made to have fewer side effects and work in smaller doses with the help of a new technique that makes drug molecules more resistant to breakdown by the human liver.
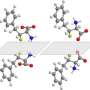
Researchers based at Princeton University reported in the journal Science that they created a synthetic enzyme that acts as a catalyst to replace certain hydrogen atoms of a drug molecule with fluorine atoms. This swap stabilizes the molecule and makes it resistant to the liver enzymes that can inactivate a drug or create toxic byproducts.
"Putting fluorine in place of hydrogen in a molecule tends to result in higher potency and lower toxicity," said first author Wei Liu, a graduate student in the laboratory of John Groves, Princeton's Hugh Stott Taylor Chair of Chemistry. Wei worked with Groves and second author Xiongyi Huang, a Princeton chemistry graduate student, as well as with Professor William Goddard III, researcher and lab director Robert Nielsen, and graduate student Mu-Jeng Cheng, all of the California Institute of Technology's Materials and Process Simulation Center.
Substituting fluorine for hydrogen changes the ability of liver enzymes to modify a drug, Groves said. Those enzymes break down medicines and other foreign substances that enter the body resulting in byproducts known as metabolites. Metabolites sometimes interact in harmful ways with liver cells and cause unwanted side effects. Fluorine reduces or eliminates the production of metabolites because the liver enzymes cannot break down the fluorinated drugs, Groves said.
"The strategy is to put fluorine at a site on the molecule where it would block metabolism by liver enzymes," Groves said. In some cases, he said, liver enzymes may not be able to break down the fluorinated drug at all, allowing more of the drug to persist in the body.
The synthetic enzyme could have uses in drug discovery and development including in improving existing drugs such as steroids, Groves explained. Steroids are used as anti-inflammatory drugs as well as in hormone-replacement therapies and birth-control pills. Steroid hormones that might be improved by fluorination include progesterone, premarin and estradiol, all of which are among the top 200 drugs in sales. The Princeton enzyme also could make it easier and less expensive to produce radioactive tracer versions of many drugs, which could be used withmedical imaging to understand how and where drugs work in the body.
Tobias Ritter, a Harvard University associate professor of chemistry, said that the Princeton catalyst's novel abilities represent "a quantum leap in the fluorination field." Ritter is familiar with the study but had no role in it.
"The most exciting advance described in the paper is the fundamental reactivity of transforming carbon-hydrogen bonds into carbon-fluorine bonds using a fluoride-oxidant mixture," Ritter said. "Not only were chemists not able to perform such reactions in the past, we are not aware of similar reactions occurring in nature."
The catalyst Liu, Groves and Huang created breaks certain carbon-to-hydrogen bonds on pharmaceutical molecules and replaces the hydrogen with fluorine. Once the catalyst was developed, the Caltech group led by Goddard created computer models to explore its actions.
The synthetic enzyme is similar in structure to a naturally occurring iron-based liver enzyme called cytochrome P450, which normally replaces hydrogen atoms with oxygen atoms. The Princeton catalyst instead uses the metal manganese as a center atom. Because the manganese catalyst mimics the behavior of human liver enzymes, the compounds created when the catalyst is used in drug development are less likely to be broken down by those natural enzymes, Groves said.
The work grew out of the Groves' lab work on cytochrome P450. In 2010, Liu and Groves reported in the Journal of the American Chemistry Society the successful development of a synthetic manganese-based P450 that could replace hydrogen with a chlorine atom instead of an oxygen atom, which made drug molecules more reactive.
Suspecting that this catalyst could replace hydrogen with fluorine as well, Liu tested different fluorine-containing materials and eventually discovered that a combination of silver fluoride and tetrabutylammonium fluoride trihydrate led to drug fluorination. Huang isolated pure crystals of the manganese catalyst and assisted with modeling the molecule using computers.
An advantage for using the catalyst in drug development is that it uses a stable form of fluorine called silver fluoride as a base material instead of fluorine gas, which reacts with numerous other atoms and can be explosive.
"We can use ordinary fluoride salts, almost like the stuff that goes into toothpaste," Groves said.
More information: The paper, "Oxidative Aliphatic C-H Fluorination with Fluoride Ion Catalyzed by a Manganese Porphyrin," was published Sept. 14 in Science. www.sciencemag.org/content/337/6100/1322.abstract
Journal information: Science
Provided by Princeton University