Searching for molecular radical's secrets of stability
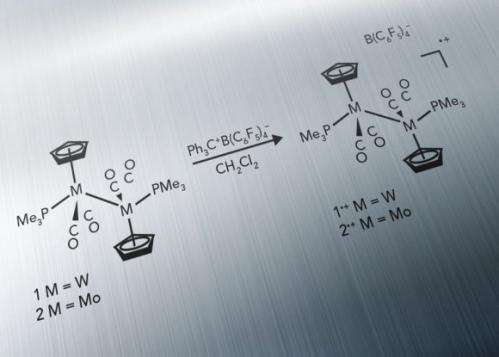
For the first time, scientists synthesized and characterized a metal-containing complex long thought to be too unstable to study. This complex piqued the interest of scientists at Pacific Northwest National Laboratory because the metals inside this metalloradical are held together by a bond between two tungsten or molybdenum atoms. The lack of strings of atoms, called ligands, around the metals is unusual, and the instability of the molecules has made them impossible to observe. Until now.
"We were happy to find that the oxidized metal-metal bonded complex could be isolated. The computational study of the molecular orbitals provides an explanation for the observation that the two metals are closer in the oxidized complex," said Dr. Morris Bullock, a PNNL scientist who worked on the study.
Proteins are often based on metalloradicals. To be able to delve into the arrangement of atoms and how they behave could open doors in the development of biofuels as well as the creation of catalysts that mimic natural proteins in creating energy and fuels.
Unlike the radical molecules that scientists knew existed, but could not observe, the new version is stable enough to be isolated and characterized. The team conducted theoretical studies, including relativistic density functional theory calculations, and experimental studies on the complex. The team benefitted from a new capability that allowed them to integrate magnetic resonance measurements and electronic structure calculations to understand the structure and dynamics of complex systems.
In characterizing the complex, researchers made two key discoveries. First, the complex contains a shorter-than-expected metal-metal bond and, as desired, the metals are not supported by bridging ligands. Second, the complex's lone electron does not favor one metal over the other. Rather, it spends equal time around the two tungsten or molybdenum atoms. This research challenges the paradigm that metal-bridging ligands are vital to stabilizing dinuclear metalloradicals.
More information: van der Eide EF, P Yang, ED Walter, T Liu, and RM Bullock. 2012. "Dinuclear Metalloradicals Featuring Unsupported Metal-Metal Bonds."Angewandte Chemie International Edition 51(33):8361-8364. DOI: 10.1002/anie.201203531
Journal information: Angewandte Chemie International Edition
Provided by Pacific Northwest National Laboratory