Researchers create new microparticles that self-assemble like atoms into molecules

Scientists have created new kinds of particles, 1/100th the diameter of a human hair, that spontaneously assemble themselves into structures resembling molecules made from atoms. These new particles come together, or "self-assemble," to form structures in patterns that were previously impossible to make and hold promise for manufacturing advanced optical materials and ceramics.
The method, described in the latest issue of the journal Nature, was developed by a team of chemists, chemical engineers, and physicists at New York University (NYU), the Harvard School of Engineering & Applied Sciences, the Harvard Department of Physics, and Dow Chemical Company.
The method is centered on enhancing the architecture of colloids—small particles suspended within a fluid medium. Colloidal dispersions are composed of such everyday items as paint, milk, gelatin, glass, and porcelain, but their potential to create new materials remains largely untapped.
Previously, scientists had succeeded in building rudimentary structures from colloids. But the ability use colloids to design and assemble complex 3-dimensional structures, which are vital to the design of advanced optical materials, has been limited. This is, in part, because colloids lack directional bonds, which are necessary to control particle self-assembly as well as to enhance complexity while maintaining the structural integrity of these creations. Such assemblies serve as the building blocks of the natural world—e.g., atoms and molecules—but they are rare in the colloidal domain.
"What this method aimed to do was to use nature's properties for atoms and apply them to the colloidal world," explained NYU chemistry professor Marcus Weck, one of the study's coauthors.
"Chemists have a whole periodic table of atoms to choose from when they synthesize molecules and crystals," added coauthor Vinothan Manoharan, Associate Professor of Chemical Engineering and Physics at Harvard. "We wanted to develop a similar 'construction set' for making larger-scale molecules and crystals."
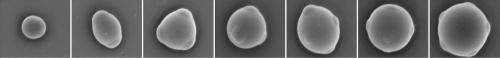
In developing colloids with such properties, the researchers engineered chemical "patches" that can form directional bonds, thus allowing for the assembly of 3-dimensional "lattices" with only a few connections between particles, an important design element for many advanced materials. Without directional bonding, such structures are unstable.
The trick was establishing bonding capabilities on the patches. The scientists did so by using single strands of DNA, which scientists at NYU and elsewhere have previously employed to organize small particles. In the method described in Nature, these strands of DNA served as "sticky ends" to which particle patches could adhere.
"What this means is we can make particles that attach only at the patches, and then we can program them so only specific kinds of particles attach at those patches," said coauthor and NYU physics professor David Pine. "This gives us tremendous flexibility to design 3-dimensional structures."
The researchers added that the specificity of DNA interactions between patches means that colloids with different properties, such as size, color, chemical functionality, or electrical conductivity, could lead to the production of new materials. These potentially include 3-dimensional electrically wired networks or photonic crystals to enhance the optical displays of a range of consumer products and to improve the speed of computer chips.
Journal information: Nature
Provided by Harvard University

















