Cellular accumulation of misfolded protein clumps may be a survival advantage rather than a liability, researchers find
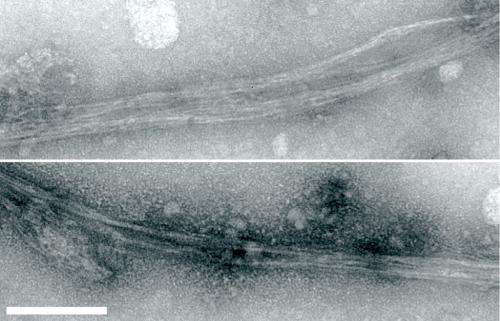
Most proteins have a single 'correct' way to fold; typically, improperly folded proteins are promptly eliminated by cells. However, certain misfolded proteins have a tendency to aggregate in dense fibrous clumps known as amyloid plaques. In humans, this accumulation is often a pathological feature, as observed in Alzheimer's or Huntington's disease.
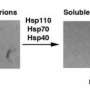
Surprising findings, however, have demonstrated that amyloid formation may also be beneficial for cells under certain conditions. They conducted an assay to identify yeast prions—proteins with the capacity to misfold in a manner that induces similar misfolding in other molecules, resulting in amyloid formation. Their screen revealed 66 candidate yeast prions, but Tanaka and colleagues focused on Mod5, a particularly interesting protein.
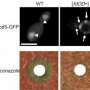
The Mod5 enzyme is normally responsible for introducing chemical modifications to the transfer RNA molecules that regulate synthesis of new proteins. The researchers demonstrated that it can also be induced to assemble into amyloid aggregates. This misfolding is also 'contagious': introduction of Mod5 amyloids into yeast cells that express only the soluble form of the protein ([mod- ] cells) gave rise to cells that instead produce the aggregating prion form of Mod5 ([MOD+] cells).
This shift proved to have important physiological consequences. Mod5 normally makes use of a chemical called DMAPP, which is also used in production of the membrane lipid ergosterol. The reduced levels of functional Mod5 in [MOD+] cells therefore leave more DMAPP available for erogsterol production. "[MOD+] cells may have thicker cell membranes, which would protect the yeast cells from attack by antifungal drugs," says Tanaka. Several experiments confirmed this protective effect, and prion-forming [MOD+] cells proved more resistant to antifungal agents such as fluconazole than their soluble [mod- ] counterparts.
This resistance comes at a cost. "[mod- ] yeast have a growth advantage in the absence of antifungal drugs," says Tanaka. As such, the [mod- ] state is selected for unless the presence of drugs makes the reduced growth rate the only alternative to poisoning. These findings demonstrate an unexpected functional importance for protein 'misfolding'. Tanaka notes that there is even evidence to suggest that the amyloids formed in Alzheimer's and other neurodegenerative diseases may accumulate as part of a protective mechanism triggered by cellular stress.
Future studies should reveal more about how this surprising defense mechanism is triggered and regulated. "We would like to learn more about how protein aggregation can regulate a cell's stress response," says Tanaka.
More information: Suzuki, G., Shimazu, N. & Tanaka, M. A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science 336, 355–359 (2012). www.sciencemag.org/content/336/6079/355.abstract
Journal information: Science
Provided by RIKEN