September 6, 2012 report
Mystery over apparent dearth of lithium 7 in universe deepens
(Phys.org)—Researchers studying the cosmos have been stumped by an observation first made by Monique and François Spite of the Paris Observatory some thirty years ago; they noted that in studying the halos of older stars, that there should be more lithium 7 than there appeared to be in the universe. Since that time many studies have been conducted in trying to explain this apparent anomaly, but thus far no one has been able to come up with a reasonable explanation. And now, new research has deepened the mystery further by finding that the amount of lithium 7 in the path between us and a very young star aligns with would have been expected shortly after the Big Bang, but doesn't take into account the creation of new amounts since that time. In their paper published in the journal Nature, Christopher Howk and colleagues suggest the discrepancy is troubling because it can't be explained with normal astrophysics models.
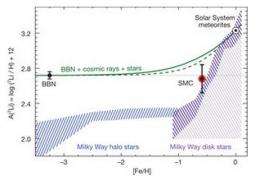
What's really bothering all the scientists working on the lithium problem is the fact that it's the only element that doesn't fit with models of how things should have come to exist right after the Big Bang. All known elements occur in amounts predicted, except for lithium 7; there's just a third as much as theorists think there should be. In trying to understand why, researchers have looked at old stars that surround the Milky Way galaxy, low mass bosons called axions, and more recently binary stars that are believed to harbor black holes. Unfortunately, such studies have only made the problem worse by suggesting that even more lithium 7 ought to be hanging around somewhere than was predicted earlier.
In this new research the team looked at one single huge young star in the Small Magellanic Cloud, or more precisely, at the spectrum measured of gas and dust through which light must travel to get from there to here, and found that the amount of lithium 7 is consistent with theories that suggest how much of the element there should have been shortly after the Big Bang, which is unsettling because scientists know that more of it should have been created between then and now. Thus, these new results only add to the mystery of where all the rest of it is, or worse, why it wasn't created in the first place as models suggest.
More information: Observation of interstellar lithium in the low-metallicity Small Magellanic Cloud, Nature, 489, 121–123 (06 September 2012) doi:10.1038/nature11407
Abstract
The primordial abundances of light elements produced in the standard theory of Big Bang nucleosynthesis (BBN) depend only on the cosmic ratio of baryons to photons, a quantity inferred from observations of the microwave background. The predicted primordial 7Li abundance is four times that measured in the atmospheres of Galactic halo stars. This discrepancy could be caused by modification of surface lithium abundances during the stars' lifetimes or by physics beyond the Standard Model that affects early nucleosynthesis. The lithium abundance of low-metallicity gas provides an alternative constraint on the primordial abundance and cosmic evolution of lithium that is not susceptible to the in situ modifications that may affect stellar atmospheres. Here we report observations of interstellar 7Li in the low-metallicity gas of the Small Magellanic Cloud, a nearby galaxy with a quarter the Sun's metallicity. The present-day 7Li abundance of the Small Magellanic Cloud is nearly equal to the BBN predictions, severely constraining the amount of possible subsequent enrichment of the gas by stellar and cosmic-ray nucleosynthesis. Our measurements can be reconciled with standard BBN with an extremely fine-tuned depletion of stellar Li with metallicity. They are also consistent with non-standard BBN.
Journal information: Nature
© 2012 Phys.org



















