Success in preparing and isolating a heavy ketone paves the way for synthesizing novel catalysts
The carbon–oxygen (C=O) double bond is an important chemical motif, particularly in compounds called ketones. Chemists expect that substituting the carbon for a heavier atom would produce 'heavy ketones', which are attractive targets to further understanding of chemical bonding and for synthesizing novel molecules. Now, Kohei Tamao at the RIKEN Advanced Science Institute in Wako, Japan, and colleagues have—for the first time—isolated a heavy ketone, where the carbon is replaced by germanium.
The researchers were motivated by the importance of ketones in both nature and industry: carbon dioxide (CO2) contains two C=O double bonds, while acetone, or nail polish remover, has only one. Ketones react reliably with numerous chemicals and are important precursors in the synthesis of pharmaceuticals. Tamao and colleagues therefore investigated replacing the carbon in ketones with heavier atoms—in this case germanium—a first step in the quest for unusual catalytic systems.
Molecules are made of atoms joined together by chemical bonds. The properties of each atom affect the strength and reactivity of the bond. Electronegative atoms, such as oxygen, pull electrons towards themselves and become negatively charged, which make the bonds highly reactive.
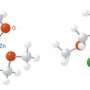
"We expected the germanium–oxygen double bonds (Ge=O) to be extraordinarily polarized because of the difference in electronegativity between the two atoms," Tsukasa Matsuo, co-author, explains. Previous attempts to make molecules with a Ge=O resulted in spontaneous combination to form oligomers—or short polymers. The researchers realized that by using bulky protecting groups, they could stabilize the heavy ketones and prevent them reacting together. These large bulky groups or atoms prevent polymerization by simply getting in the way of other chemicals that otherwise react with them.
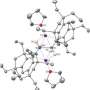
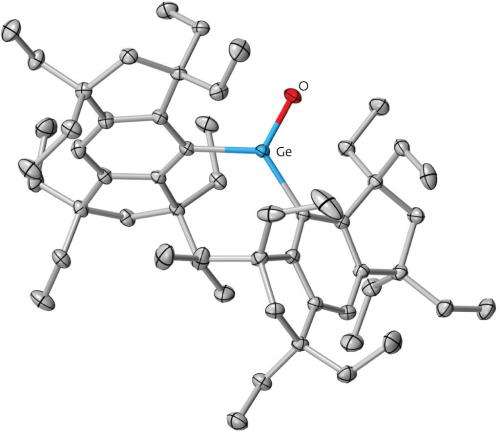
Liangchun Li, another co-author, isolated a germanium ketone, or germanone, as colorless crystals by selecting a bulky ligand known as Eind (Fig. 1). With the heavy ketone in hand, the team then compared and contrasted them to normal ketones and investigated both their physical and reactive properties.
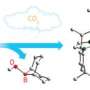
The researchers' experiments showed that germanones are more highly reactive than regular ketones. For instance, they react readily with carbon dioxide and water at room temperature whereas regular ketones do not. Computer simulation models have confirmed this reactivity: they indicate that the Ge=O bond is almost completely charge separated.
The next step for the researchers is to create a silicon–oxygen double bond. "Thanks to this chemistry, we have a chance to develop a new catalytic system involving germanones, such as oxygen atom transfer reactions," Matsuo notes.
More information: Li, L., Fukawa, T., Matsuo, T., Hashizume, D., Fueno, H., Tanaka, K. & Tamao, K. A stable germanone as the first isolated heavy ketone with a terminal oxygen atom. Nature Chemistry 4, 361–365 (2012). www.nature.com/nchem/journal/v … /abs/nchem.1305.html
Journal information: Nature Chemistry
Provided by RIKEN