New substances 15,000 times more effective in destroying chemical warfare agents
In an advance that could be used in masks to protect against nerve gas, scientists are reporting development of proteins that are up to 15,000 times more effective than their natural counterpart in destroying chemical warfare agents. Their report appears in ACS' journal Biochemistry.
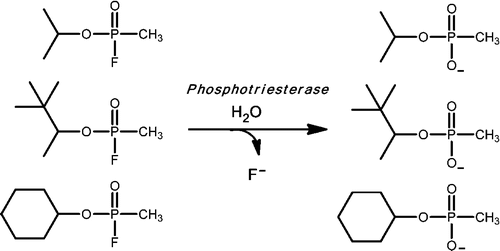
Frank Raushel, David Barondeau and colleagues explain that a soil bacterium makes a protein called phosphotriesterase (PTE), which is an enzyme that detoxifies some pesticides and chemical warfare agents like sarin and tabun. PTE thus has potential uses in protecting soldiers and others. Natural PTE, however, works against only one of the two molecular forms of these chemical warfare agents, and it happens to be the less toxic form. The scientists thus set out to develop new versions of PTE that were more effective against the most toxic form.
To improve the enzyme's activity, Raushel and colleagues used an approach called "directed evolution." This technique imitates the way natural selection leads to improved forms of the biochemical substances in living things. In using directed evolution, the team made small random changes to the natural enzyme's chemical architecture and then tested resulting mutant enzymes for their ability to break down nerve agents. They isolated several mutants that fit the bill, including one that proved to be 15,000 times more effective than the natural enzyme.
More information: “Enzymes for the Homeland Defense: Optimizing Phosphotriesterase for the Hydrolysis of Organophosphate Nerve Agents” Biochemistry, Article ASAP. DOI: 10.1021/bi300811t
Abstract
Phosphotriesterase (PTE) from soil bacteria is known for its ability to catalyze the detoxification of organophosphate pesticides and chemical warfare agents. Most of the organophosphate chemical warfare agents are a mixture of two stereoisomers at the phosphorus center, and the SP-enantiomers are significantly more toxic than the RP-enantiomers. In previous investigations, PTE variants were created through the manipulation of the substrate binding pockets and these mutants were shown to have greater catalytic activities for the detoxification of the more toxic SP-enantiomers of nerve agent analogues for GB, GD, GF, VX, and VR than the less toxic RP-enantiomers. In this investigation, alternate strategies were employed to discover additional PTE variants with significant improvements in catalytic activities relative to that of the wild-type enzyme. Screening and selection techniques were utilized to isolate PTE variants from randomized libraries and site specific modifications. The catalytic activities of these newly identified PTE variants toward the SP-enantiomers of chromophoric analogues of GB, GD, GF, VX, and VR have been improved up to 15000-fold relative to that of the wild-type enzyme. The X-ray crystal structures of the best PTE variants were determined. Characterization of these mutants with the authentic G-type nerve agents has confirmed the expected improvements in catalytic activity against the most toxic enantiomers of GB, GD, and GF. The values of kcat/Km for the H257Y/L303T (YT) mutant for the hydrolysis of GB, GD, and GF were determined to be 2 × 106, 5 × 105, and 8 × 105 M–1 s–1, respectively. The YT mutant is the most proficient enzyme reported thus far for the detoxification of G-type nerve agents. These results support a combinatorial strategy of rational design and directed evolution as a powerful tool for the discovery of more efficient enzymes for the detoxification of organophosphate nerve agents.
Journal information: Biochemistry
Provided by American Chemical Society