August 20, 2012 feature
Into the breach: Transporting molecular cargo through algal cell walls
(Phys.org) -- Algae constitute a widespread, highly varied group of primarily photosynthetic organisms found in many ecological niches. The roughly estimated 300,000 species range in size and complexity from small to very large, and unicellular to multicellular, respectively. Moreover, their properties are equally diverse in terms of applications, having a potentially unique role in medical, materials, energy, bioremediation, and synthetic biological research. That being said, methods for investigating and making use of algal biology have been slow to emerge. Recently, however, scientists at Stanford University and Lawrence Berkeley National Laboratory have devised a general-purpose molecular technique that allows cargoes of various sizes to be transported through the algal cell wall and membrane, thereby accessing the intracellular algal space.
Stanford University Bergstrom Professor of Chemistry Prof. Paul A. Wender, Lawrence Berkeley National Laboratory scientists Joel M. Hyman, and Bahram Parvin, and Stanford researchers Erika I. Geihe and Brian M. Trantow encountered a series of challenges in designing and implementing their method of variable-scale algal cell molecular transport and delivery. “We reported for the first time in 2000, based on our systematic reverse engineering of the Tat nine-mer, a nonapeptide sequence from the protein HIV Tat, “ Wender tells Phys.org, “that the number and spatial array of guanidinium groups is what allows both the Tat protein and Tat peptide to enter mammalian cells. At the time we coined the term molecular transporters, as we realized from our research that this ability to enter cells was not a function of the peptidic backbone but rather the number of guanidinium groups and their spacing. This led to the design of peptoid transporters, non-peptidic transporters, dendrimers and many other guanidinium-rich transporters capable of carrying molecules into cells,” Wender explains.
Much of this earlier work focused on getting transporters through the plasma membrane of mammalian cells. At that time, they also showed that these guanidinium-rich transporters will ferry a variety of cargos including peptides, metals, probes, proteins, and nucleic acids (siRNA) into cells. In addition, these transporters were also shown to transport cargo across other barriers such as the skin.
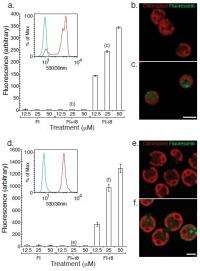
“Algae presented a new challenge as they not only have a membrane barrier but also, for many types of algae, a cell wall,” Wender continues. “Thus, our initial task was simply to see if our lead transporter octaarginine, when attached to a fluorescent tag such as fluorescein, would get through both the cell wall and cell membrane of algae.” While the synthesis of this oligoarginine-fluorescein conjugate follows directly from their previous studies, recording uptake of this conjugate into algae proved to be more difficult than working with mammalian cells – because, Wender exclaims, the algae move! Their solution (which Wender unabashedly describes as clever): make small wells for the algae to dock, preventing their motion during the process of recording images.
A significant benefit, Wender adds, was that making non-covalent protein complexes – which often can be quite difficult – proved to be relatively straightforward. “This has value since one needn’t invest in making covalent conjugates – itself often a challenging process,” he adds. “However, showing that the proteins have functional activity was more complex, as both uptake and function needed to be achieved. While technically more demanding, the process worked impressively well. It’s great to be working with such creative and determined coworkers.”
Wender also points out that their findings impact several areas of research. “Firstly, in algal research our ability to study and manipulate algae is determined by our ability to introduce molecules into algae that could report back or create change. Some molecules do this on their own, but the vast majority of molecules do not pass through the algal cell wall and membrane.” Mechanical and physical methods – for example, biolistics (gene guns), sonication, electroporation, and glass beads – have been developed to circumvent this limitation. However these are often expensive, encounter reproducibility problems and/or are not readily scaled.
“Shooting DNA coated particles into algae is an important technique but it is well quite literally hit-and-miss,” Wender says. “However, we’re now reporting a molecular method in which algae are incubated with a probe connected to a molecular transporter, which enables the resultant conjugate to enter cells, and moreover show that this works for both small molecules and large proteins.” In other words, agents that would otherwise not enter cells are now carried into cells by the molecular transporters. This opens a wide range of chemicals that could be used to study algal function and to use algae in drug discovery, as tools for research including energy research, as agents for bioremediation or as biosensors.
Secondly, Wender continues, their findings impact the use of algae as photoautotrophic tools for synthetic biology. “Being able to transform algae allows one to control what they produce and the quantity of what they produce. Algae already produce products of commercial value – and this new technology could be used to enhance those processes.” Of equal imports, he adds, their new technology could be used to induce algae to produce new materials of potential value in research, materials science, and even therapy.
Finally, Wender points out that their results will enhance the understanding of biological barriers in the life sciences – specifically, agricultural and medical research. “Biological barriers are of great importance in many areas of science. If a drug, probe or agent does not enter a cell we cannot begin to understand the inner workings of a cell or manipulate it for diagnostic or therapeutic ends. This study now provides a powerful tool to study and manipulate algae.” The cell wall barrier is also found in plants, and so this research has ramifications in agriculture and plant sciences. “We now have a new and molecular way to ferry chemicals across cell walls. We can now communicate with algae.”
In terms of planned next steps in their research, Wender concludes, algae represent model organisms for drug discovery, for sensing, for energy research and for bioremediation. “Algae are motile, and therefore can gather and concentrate pollutants. Learning how to control that process is one of many directions with environmental ramifications, and learning how they process drug candidates could be a great way to screen for future therapeutics.”
More information: A molecular method for the delivery of small molecules and proteins across the cell wall of algae using molecular transporters, PNAS August 14, 2012 vol. 109 no. 33 13225-13230, doi:10.1073/pnas.1202509109
Journal information: Proceedings of the National Academy of Sciences
Copyright 2012 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.