'Trophy molecule' breakthrough
Experts at The University of Nottingham are the first to create a stable version of a ‘trophy molecule’ that has eluded scientists for decades.
In research published in journal Science, the team of chemists at Nottingham has shown that they can prepare a terminal uranium nitride compound which is stable at room temperature and can be stored in jars in crystallized or powder form.
Previous attempts to prepare uranium-nitrogen triple bonds have required temperatures as low as 5 Kelvin (-268 °C) — roughly the equivalent temperature of interstellar space — and have therefore been difficult to work with and manipulate, requiring specialist equipment and techniques.
The breakthrough could have future implications for the nuclear energy industry — uranium nitride materials may potentially offer a viable alternative to the current mixed oxide nuclear fuels used in reactors since nitrides exhibit superior high densities, melting points, and thermal conductivities and the process the scientists used to make the compound could offer a cleaner, low temperature route than methods currently used.
The research has been led by Dr Stephen Liddle in the School of Chemistry and much of the practical work was completed by PhD student David King. The work was also supported by colleagues at the University of Manchester.
Uranium nitrides are usually prepared by mixing dinitrogen or ammonia with uranium under high temperatures and pressures. Unfortunately, however, the harsh reaction conditions used in the preparation introduces impurities which are difficult to remove. In recent years scientists have therefore focussed their attention on using low temperature, molecular methods but all previous attempts resulted in bridging, rather than the target terminal, nitrides.
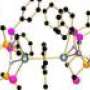
The Nottingham team’s method involved using a very ‘bulky’ nitrogen ligand (an organic molecule bonded to a metal) to wrap around the uranium centre and to create a protective pocket in which the nitride nitrogen can sit. The nitride was stabilised during the synthesis by the presence of a weakly bound sodium cation (positively charged ion) which blocked the nitride from reacting with any other elements. In the final stage, the sodium was gently teased away, removing it from the structure and leaving the final, stable uranium nitride triple bond.
Dr Liddle said: “The beauty of this work is its simplicity — by encapsulating the uranium nitride with a very bulky supporting ligand, stabilising the nitride during synthesis with sodium, and then sequestering the sodium under mild conditions we were able to at long last isolate the terminal uranium nitride linkage.”
He added: “A major motivation for doing this work was to help us to understand the nature and extent of the covalency in the chemical bonding of uranium. This is fundamentally interesting and important because it could help in work to extract and separate the 2 to 3 per cent of the highly radioactive material in nuclear waste.”
The research was supported by the UK National Electron Paramagnetic Resonance (EPR) Facility, funded by the Engineering and Physical Sciences Research Council and based in the Photon Science Institute at The University of Manchester. The uranium-nitride contains an unpaired electron and by using EPR spectroscopy it was found that it behaves differently from similar compounds prepared at Nottingham.
Professor Eric McInnes, from The University of Manchester said: “EPR spectroscopy can give detailed information about the local environment of unpaired electrons, and this can be used to understand the electronic structure of the uranium ion in this new nitride. It turns out that the new nitride behaves differently from some otherwise analogous materials, and this might have important implications in actinide chemistry which is of vital technological and environmental importance in the nuclear fuel cycle.”
Journal information: Science
Provided by University of Nottingham