A new tool for molecular architects
A team of chemists at the University of Geneva have finally demonstrated the utility of the rare halogen bond which acts as a anions transporter.
Professor Stefan Matile does not mention whether, as a child, he was a big fan of Lego. However, as an adult, he is fascinated by the game of molecular construction. Using the rules of chemistry, it is possible to artificially assemble all kinds of molecules and even nanomachines.
However, chemists in this area feel their creativity constrained by the small number of bond types available for binding atoms and molecules. Such is the case for this UNIGE and NCCR Chemical Biology chemist who decided several years ago to flush out new kinds of bonds. "The literature murmured about the existence of a possible halogen bond, particularly in thyroid gland biology," explains the specialist. "It was said to be very similar to the hydrogen bond except for an important detail: while the hydrogen bond (hydrophilic) works only in water, the halogen bond (hydrophobic) feels at ease in fatty environments."
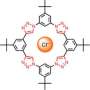
To highlight this bond and its potential usefulness, Stefan Matile's team sought to compose the smallest molecular system possible. They bonded a carbon atom and an iodine atom (member of the halogen family) to establish that in so doing they created an imbalance in the distribution of electrons orbiting the iodine nucleus. This results in an excess of negative charge on one side and a negative charge deficit on the other. This deficit acts as a positive charge, now capable of interacting with anions (negatively charged atoms).
The Geneva team's biggest contribution is to have demonstrated that this system can transport anions across a phospholipid bilayer membrane, similar to that found in our cells.
"This halogen bond acts somewhat like a buoy and allows anions to swim across the fatty interior of the membrane," adds Stefan Matile. "This is obviously a very interesting function for NCCR Chemical Biology, one of whose goals is to discover new ways to penetrate cells without damaging them."
This discovery is currently situated in the field of fundamental science, but could very quickly find applications, especially in the medical field. Some diseases are in fact linked to the inability of some organs to allow vital ions across cell membranes.
Provided by University of Geneva