SBU researchers discover significant water anomaly

(Phys.org) -- A team of researchers from the Stony Brook University Department of Physics & Astronomy along with colleagues from the Department of Condensed Matter Physics at Universidad Autónoma de Madrid (UAM) in Spain, explain a puzzling water anomaly in a paper published in the May 9 edition of Physical Review Letters entitled, “Anomalous Nuclear Quantum Effects in Ice.” The work details an anomaly – a deviation from the common form – of water ice that has been largely neglected and never before explained.
“We believe that our study explains a rare, seldom mentioned property of ice which should be included in the list of water anomalies as an example in which quantum effects are anomalous and increase with temperature,” said Marivi Fernandez-Serra, an assistant professor in the Department of Physics & Astronomy at Stony Brook, who collaborated with three UAM professors and Stony Brook Professors Philip Allen and Peter Stephens, and PhD student Betuk Pamuk.
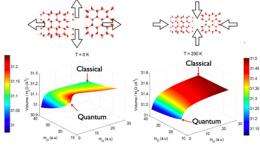
In this contribution, the researchers show that the volume of water (H2O) ice depends on the quantum “zero-point” motion of the H and O atoms in an opposite way from “normal” materials. Crystals shrink as they are cooled, but because of “zero-point” motion, shrinking stops before reaching temperatures of absolute zero. This effect is a result of the Heisenberg Uncertainty Principle, which states that there is a fundamental limit on the accuracy with which certain pairs of physical properties can be simultaneously identified - the more precisely one property is measured, the less precisely the other can be controlled, determined or known.
Less massive atoms are more “quantum,” with more zero-point energy. Lighter nuclei need more room to move than heavier nuclei, which translates into larger crystals. At high temperatures, quantum effects become less important, so the volume differences decrease with temperature, noted Fernandez-Serra. The opposite occurs with ice. D2O (deuterated or heavy water) occupies more volume than H2O molecules, a difference that increases with temperature. “In order to access and measure quantum mechanical effects in matter, we usually need to go to very low temperatures, but in water ice some zero-point effects actually become more relevant as the temperature increases,” said Fernandez-Serra.
The theoretical model proposed, which is backed by careful computational modeling and an X-ray diffraction experiment at Brookhaven National Laboratory’s National Synchrotron Light Source, attributes the effect to the peculiar nature of the hydrogen bond. “In water, quantum mechanics manifests in a very striking way,” said Fernandez-Serra. “Its effects are more dominant with increasing temperature, which is rather unexpected.”
Journal information: Physical Review Letters
Provided by Stony Brook University


















