May 17, 2012 report
Electron hopping in graphene oxide leads to highly sensitive sensing
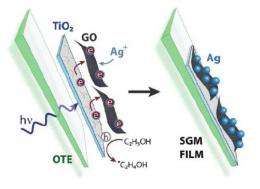
(Phys.org) -- Graphene has many promising applications on its own, but pairing the two-dimensional material with the semiconductor titanium dioxide (TiO2) extends its capabilities even further. A team of chemists at the University of Notre Dame in Notre Dame, Indiana, has demonstrated that graphene oxide (GO)-TiO2 films, when illuminated, cause electrons to hop from one side of the film to the other. When adding silver ions to the picture, this electron hopping can create films that have a semiconductor on one side of the GO and metal on the other. The resulting semiconductor-graphene-metal (SGM) films could serve as highly sensitive chemical sensors.
The researchers, led by Prashant Kamat at the Chemistry & Biochemistry and Radiation Laboratory at the University of Notre Dame, have published their study on the new graphene-based films in a recent issue of The Journal of Physical Chemistry Letters. The work was supported by the Office of Basic Energy Sciences, Department of Energy.
The study builds on earlier research that has shown that GO can function as an electron shuttle due to its ability to transport electrons across its surface, with potential applications in batteries, photovoltaics, and catalysis.
Here, the researchers have shown that photogenerated electrons from TiO2 can be captured by GO and transported through its sp2 network (the “chicken wire” structure), perpendicular to its two-dimensional plane. By having all electrons on one side, the film offers the ability for side-selective deposition of silver nanoparticles, while the semiconducting TiO2 nanoparticles remain on the opposite side.
“The conducting properties of single-to-few-layer graphene sheets deposited on various substrates have been well studied,” Kamat told Phys.org. “Usually the transport of electrons occurs within the 2-D plane. By keeping the semiconductor nanoparticles on one side, we were able to carry out reduction of silver ions on the opposite side of the graphene oxide film. This process is the direct demonstration of electron hopping in a chemically exfoliated graphene oxide film.”
To create the composite film, the researchers started by depositing single-layer graphene oxide onto a TiO2 film using electrophoretic deposition. In this method, they pasted the TiO2 film on an electrode, and then submerged it and a second electrode in ethanol containing GO sheets. Under an applied electric field, the negatively charged GO sheets moved toward and attached to the TiO2 film on the positive electrode.
To deposit the metal nanoparticles, the researchers placed the TiO2-GO films in ethanol containing silver ions. Under UV illumination, the TiO2 photogenerated electrons, which were then transferred to the GO and shuttled to the opposite side, where they were readily available for reducing the silver ions into silver nanoparticles.
The researchers found that they could control the size of the silver nanoparticles on the film by controlling the irradiation time, with longer irradiation times resulting in larger nanoparticles. They explain that loading lots of small silver nanoparticles is important for making highly active sensors and catalysts.
To test its usefulness as a sensor, the researchers placed an SGM film in a solution containing a porphyrin target in nanomolar concentrations. They found that, when the surface plasmon resonance of the silver nanoparticles interacts with the porphyrin, it amplifies the target Raman signal, which indicates the presence of the porphyrin.
The researchers predict that the graphene layer should also interact with the target molecules, which should amplify the Raman signal further and allow for an even higher sensitivity of various target molecules at very low concentrations. This sensing ability has a wide variety of applications.
“There are many contaminants in air and drinking water that need to be detected at very low level concentrations,” Kamat said. “SERS (Surface Enhanced Raman Spectroscopy) is a useful technique that makes use of small silver nanoparticles to increase the sensitivity limit of detection of chemicals. The semiconductor-graphene-metal film prepared in the present study has two distinct advantages: First, the size and loading of metal particle deposition on the graphene oxide film can be controlled through illumination of TiO2. And second, the graphene film enables adsorption of chemical contaminants from solution, thus enabling higher local concentration near the metal particle surface.
“Another potential application is in the area of photocatalytic generation of solar fuels. For example, having semiconductor nanoparticles on one side of a graphene sheet and a metal catalyst on the other side, one can create a hybrid assembly that can selectively split water into oxygen and hydrogen.”
As Kamat explained, these applications will guide the researchers’ future work.
“We are currently working towards the detection of environmental contaminants at sub-nanomolar concentration,” he said. “Careful control of metal size and loading will be the key to optimize strips for testing water quality. We are also exploring ways to design hybrid assembly for photocatalytic production of solar fuels.”
More information: Ian V. Lightcap, et al. “Electron Hopping Through Single-to-Few-Layer Graphene Oxide Films. Side-Selective Photocatalytic Deposition of Metal Nanoparticles.” The Journal of Physical Chemistry Letters. DOI: 10.1021/jz3004206
© 2012 Phys.Org




















