Efficient preparation of a set of potential glycosidase inhibitors
(Phys.org) -- In many biological and pathological processes, glycosidase enzymes attack glycosidic bonds in carbohydrates, glycoproteins, and glycolipids. The ability to modify or block these processes by specific glycosidase inhibitors forms the basis for their potential use in the treatment of viral infections, cancer, and genetic disorders.
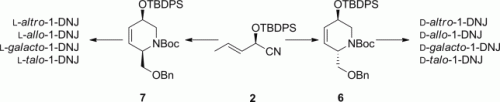
A Dutch team led by Herman S. Overkleeft has now developed a method that allows the synthesis of 8 of the 16 possible configurational isomers of the inhibitor candidate deoxynojirimycin, which will allow comprehensive medicinal chemistry screening of this library. As the scientists report in the European Journal of Organic Chemistry, their technique requires the use of a common precursor to prepare all eight compounds of biological interest.
Deoxynojirimycin and its derivatives have been long pursued by organic and medicinal chemists as a result of their potential as glycosidase inhibitors. Many groups now pursue these compounds for their application in the treatment of genetic disorders and type II diabetes. Consequently, many synthetic studies on deoxynojirimycins have appeared and continue to appear; however, synthetic strategies that allow different configurational isomers to be prepared in a concise fashion are scarce. This synthesis of such a library is important so that the compounds can be studied side by side. This technique can give chemists important insight into which structural features lead to higher levels of biological activity.
The scientists' procedure involves the use of a common cyanohydrin as the starting material, which is easily accessible in large quantities. The cyanohydrin is then transformed into cyclic building blocks from which the individual isomers can be assembled by using typical organic transformations. This work complements the large body of literature on the synthesis of 1-deoxynojirimycin derivatives with the distinguishing feature that eight stereoisomers of this important class of glycosidase inhibitors can be derived from a common precursor in an efficient manner. This team is therefore well on its way to helping scientists screen a diverse range of potential drugs that may lead to the treatment of important diseases.
More information: Herman S. Overkleeft, Synthesis of Eight 1-Deoxynojirimycin Isomers from a Single Chiral Cyanohydrin, European Journal of Organic Chemistry, dx.doi.org/10.1002/ejoc.201200377
Journal information: European Journal of Organic Chemistry
Provided by Wiley