Designing a dye you can count on
Natural substances such as chlorophyll and the heme pigment of red blood cells contain colorful molecules known as porphyrins. They owe their exceptional visual characteristics to a ‘macrocyclic’ chemical structure that links several small rings together into a highly conjugated, aromatic framework. However, chemists who have synthesized porphyrin derivatives have sometimes found that this aromaticity—and any associated optical absorptions—simply disappears.
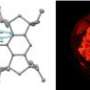
Now, a research team led by Atsuya Muranaka and Masanobu Uchiyama at the RIKEN Advanced Science Institute, Wako, reports a new way to manipulate the peculiar aromatic properties of macrocycles. The team has found that the aromaticity of a porphyrin-type molecule called hemiporphyrazine can be switched on and off by altering the compound’s electron count. This creates a dye with tunable optical absorption of near-infrared light—a type of radiation critical to applications involving organic solar cells and photodynamic cancer therapies.
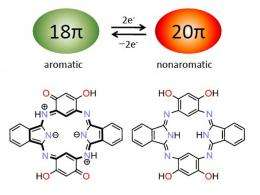
Conjugated molecules exhibit aromatic properties only when their number of so-called ‘pi’ electrons is a multiple of the formula 4n+2, where n is an integer. For example, porphyrin rings with 18 pi-electrons are stable and can share electrons aromatically, making them responsive to light. But a porphyrin with 20 pi-electrons readily gives up two electrons and returns to the favored aromatic state.
Hemiporphyrazines, however, are an unusual kind of macrocycle: their particular combination of carbon and nitrogen double bonds produces a non-aromatic structure that is thermally stable with 20 pi-electrons. Despite the promising material characteristics of these porphyrin analogues, their non-aromatic nature currently limits their usefulness. “From a theoretical point of view, it seems easy to take hemiporphyrazines down to 18 pi-electrons,” notes Muranaka. “But so far, no one has succeeded in doing this experimentally.”
The researchers solved this problem by putting four hydroxyl (OH) atoms into hemiporphyrazine to facilitate a redox reaction (Fig. 1). Mixing this compound with a strong oxidizing reagent caused two OH units to lose an electron and turn into double-bonded oxygen atoms, transforming hemiporphyrazine into an aromatic 18 pi-electron system. Consequently, the dye displayed intense near-infrared optical absorption peaks where none existed before.
The team reverted its hemiporphyrazine to 20 pi-electrons by mixing it with a reducing agent. This reversible system is sure to interest developers of ‘on-demand’ opto-electronic materials. Muranaka says that the next step is to prepare a 22 pi-electron hemiporphyrazine—a new aromatic species that quantum calculations predict would have similar or stronger near-infrared absorption bands.
More information: Muranaka, A., et al. [18]/[20]π hemiporphyrazine: a redox switchable near-infrared dye. Journal of the American Chemical Society 134, 190–193 (2012).
Journal information: Journal of the American Chemical Society
Provided by RIKEN