April 10, 2012 feature
Particles magnetically 'click' to form superstructures
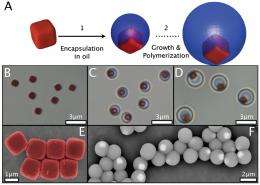
(Phys.org) -- Geomag, the popular children's toy, contains small metal spheres that can be magnetically connected with a click to build a variety of towers, bridges, and sculptures. In a new study, scientists have done something similar on the microscale: they've created a new class of spherical colloids that have tiny magnetic patches embedded beneath their surfaces. In the absence of a magnetic field, the colloidal particles can spontaneously form clusters of controlled size and shape. With the application of an external magnetic field, the clusters can unbind and change their geometry, allowing the structures to reconfigure themselves independent of the chemical conditions of the environment.
The researchers, Stefano Sacanna and David J. Pine of New York University and Laura Rossi of Utrecht University in Utrecht, The Netherlands, have published their study on the magnetic colloids in a recent issue of the Journal of the American Chemical Society.
“From the cover of your iPad, which clicks magnetically and snugly fits into place, to kids' construction toys, magnetic forces precisely direct objects to bind or align with each other,” Sacanna told Phys.org. “We thought of using this 'invisible' glue to regulate the way microscopic objects assemble. In particular, because magnetic forces are permanent (do not degrade in time) and virtually unaffected by the local chemical environment, this new binding and recognition mechanism between colloidal building blocks offers a large design freedom to material scientists.”
The spherical colloids start out as cubes, each of which contains a tiny piece of magnetic iron oxide. The researchers encapsulated the cubes inside silicon oil droplets so that the cubes are trapped at the interface by surface tension. In this arrangement, one face of the cube extends outside the droplet so that it's exposed to the surrounding water, forming a surface inhomogeneity that the scientists call a “magnetic patch.” In the final step, the oil droplets are hardened into solid colloids.
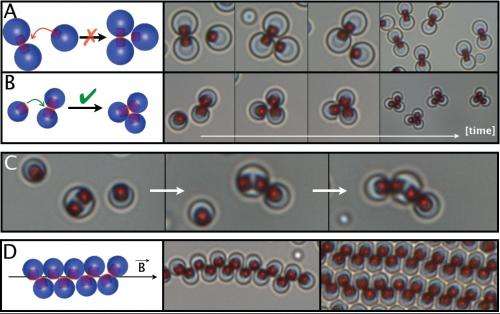
In their experiments, the researchers demonstrated that these new colloidal particles can interact with each other in various ways. Generally, the magnetic attraction between particles has a short range, which is offset by the long-range repulsion of the particles' electric forces. As a result, the particles only connect when they are already close together. However, the researchers could decrease the particles' electric repulsion by adding salt to the water, which effectively increases the particles' magnetic binding energy.
As the researchers demonstrated, in the absence of an applied magnetic field, two or three particles in close proximity self-assemble into dimers or trimers. By synthesizing particles with multiple magnetic patches, the researchers could enable larger, more complex structures to self-assemble due to the multiple binding points. The researchers also adapted the inter-particle binding mechanism to bind particles onto magnetically patterned surfaces.
In these cases, an external magnetic field applied perpendicular to surface of the water containing the particles can change the orientation of the particles' magnetic dipoles. This change causes the particles to rotate and repel each other, and the clusters to unbind. When a homogenous magnetic field is applied, large clusters of particles with multiple magnetic patches reorganize themselves into linear chains.
With the ability to control the size of the particles, the size and number of the magnetic patches, and the external magnetic field, this self-assembly method could have a variety of applications. One potential use is drug delivery, in which a particle and its cargo is delivered to a specific location. An advantage of the magnetic method compared to chemical methods of particle assembly is that magnetic particles are independent of most environmental changes such as temperature, pH, and solvent composition.
“Magnetic bonds can form, break and rearrange as many times as we want (again, think about Geomag toys and on how many complex structures you can build with only a few simple elementary units),” Sacanna said. “By applying this principle to colloidal particles, we can envision the creation of colloidal fluids that, upon an external stimulus (for example, the addition of salt), can self-organize into functional materials with microscopic architectures that are enforced by the formation of directional magnetic bonds. The use of external magnetic fields would add the possibility to easily break and reset such architectures. Other than smart reconfigurable materials, these magnetic colloids could find applications as a 'transporting vehicle' in drug delivery as the particles can 'sense' magnetic fields and autonomously bind on magnetic targets.”
In the future, the researchers plan to improve the particles' control as well as their “intelligence.”
“We are currently working on fabricating particles with a higher number of magnetic patches and ultimately we would like to control the orientation of the magnetic dipole in the patch to realize colloids with a controllable 'valence number,” Sacanna said. “This would allow us to assemble colloidal structures simply by mixing particles in a stoichiometric ratio. Finally, we would like to make our particles even 'smarter' by combining our magnetic patches with other classical surface modifications such as DNA coatings, chemical patches, electrostatic charges, etc.”
More information: Stefano Sacanna, et al. “Magnetic Click Colloidal Assembly.” Journal of the American Chemical Society. DOI: 10.1021/ja301344n
Journal information: Journal of the American Chemical Society
Copyright 2012 Phys.Org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.

















