March 23, 2012 report
Safer way to make diazomethane developed
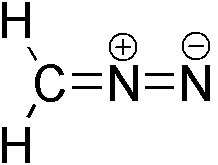
(PhysOrg.com) -- Diazomethane is a toxic, explosive reagent prepared as needed in laboratories, where it is commonly used in cyclopropanation, but its explosive nature prevents it being used widely on an industrial scale. Now, scientists in Switzerland have developed a new method of synthesising diazomethane that is much safer and therefore could lead to much wider use.
Diazomethane (CH2N2) is a toxic yellow gas at 25 degrees Celsius, and is usually used in solution in ether. It is a useful reagent for many reasons, such as its ability to be converted to carbene, which is used in the synthesis of cyclopropanes. It is usually prepared as needed in the lab by hydrolysis of a precursor such as diazald (N-methyl-N-nitroso-p-toluenesufonamide), but becomes explosive during the processes of purifying and isolating it. Safety precautions such as preparing the chemical in a fume hood or behind a blast shield, avoiding ground glass joints, wearing thick protective gloves, avoiding any sharp edges or scratches on the glassware, and so on, are employed during the synthesis to protect the scientists from potential explosions.
Researchers Erick Carreira and Bill Morandi of the Laboratory of Organic Chemistry at ETH in Zurich have developed a new method for generating diazomethane in situ that uses a catalyst and avoids the need for the dangerous isolation process and generation of large volumes of the explosive chemical. The catalyst is an iron porphyrin complex and is used to catalyze the cyclopropanation of olefins such as styrenes, dienes, and enynes in an aqueous solution of potassium hydroxide (6M, KOH). Since olefins are immiscible with water, they create a safe biphasic environment for the formation of the intermediate, diazomethane, which is constantly generated and immediately consumed in the reaction. The catalyst remains within the olefin phase.
The new method is safer for the chemists because there is a much lower risk of explosion since the diazomethane is consumed as soon as it is created, and large volumes do not accumulate. There is also a much lower chance of exposure to the toxic gas.
In the paper, published in Science, Carreira and Morandi suggest the method could also be used in the synthesis of other toxic or explosive reagents, provided they can be prepared in aqueous solutions.
More information: Iron-Catalyzed Cyclopropanation in 6 M KOH with in Situ Generation of Diazomethane, Science 23 March 2012: Vol. 335 no. 6075 pp. 1471-1474, DOI: 10.1126/science.1218781
ABSTRACT
Diazomethane is a common and versatile reagent in organic synthesis whose broader use is generally impeded by its explosiveness and toxicity. Here we report that a simple iron porphyrin complex catalyzes the cyclopropanation of styrenes, enynes, and dienes under the demanding conditions [aqueous 6 molar potassium hydroxide (KOH) solution, open to air] necessary for the in situ generation of diazomethane from a water-soluble diazald derivative. A biphasic reaction medium arising from the immiscibility of the olefin substrates with water appears essential to the overall efficiency of the process. The work we describe highlights an approach to catalysis with untoward reactive intermediates, in which the conditions for their generation under operationally safe regimes dictate catalyst selection.
Journal information: Science
© 2011 PhysOrg.com















