Iron is key to reversing global warming, Nature research shows
Canada defines itself as a nation that stretches from coast to coast to coast. But can we keep those coasts healthy in the face of climate change? Yves Gélinas, associate professor in Concordia's Department of Chemistry and Biochemistry, has found the solution in a surprising element: iron.
In a study published in Nature, Gélinas — along with Concordia PhD candidate Karine Lalonde and graduate Alexandre Ouellet, as well as McGill colleague Alfonso Mucci — studies the chemical makeup of sediment samples from around the world ocean to show how iron oxides remove carbon dioxide from our atmosphere.
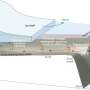
"People around the planet are fighting to reduce the amount of CO2 pumped into the atmosphere in the hopes of reducing climate change. But when it comes to getting rid of the CO2 that's already there, nature herself plays an important role," Gélinas explains. CO2 is removed from the atmosphere and safely trapped on the ocean floor through a natural reaction that fixes the molecule to organic carbon on the surface of large bodies of water.
How exactly does that fixation process work? "For well over a decade, the scientific community has held onto the hypothesis that tiny clay minerals were responsible for preserving that specific fraction of organic carbon once it had sunk to the seabed," explains Mucci, whose related research was picked as one of the top 10 Scientific Discoveries of the year by Québec Science. Through careful analysis of sediments from all over the world, Gélinas and his team found that iron oxides were in fact responsible for trapping one fifth of all the organic carbon deposited on the ocean floor.
With this new knowledge comes increased concern: iron oxides are turning into what might be termed endangered molecules. As their name suggests, iron oxides can only form in the presence of oxygen, meaning that a well-oxygenated coastal ecosystem is necessary for the iron oxides to do their work in helping to remove carbon dioxide from the atmosphere. But there has been a worrying decrease in dissolved oxygen concentrations found in certain coastal environments — and this trend is expanding. Locations once teeming with life are slowly becoming what are known as "dead zones" in which oxygen levels in the surface sediment are becoming increasingly depleted. That familiar culprit, man-made pollution, is behind the change.
Major rivers regularly discharge pollutants from agricultural fertilizers and human waste directly into lake and coastal environments, leading to a greater abundance of plankton. These living organisms are killed off at a greater rate and more organic carbon is sinking to the bottom waters, causing even greater consumption of dissolved oxygen. This makes the problem of low dissolved oxygen levels even worse. If the amount of oxygen in an aquatic environment decreases beyond a certain point, iron oxides stop being produced, thus robbing that environment of a large fraction of its natural ability to extract carbon dioxide from the atmosphere.
But there is hope. "This study also represents an indirect plea towards reducing the quantities of fertilizers and other nutrient-rich contaminants discharged in aquatic systems" explains Lalonde, who Gélinas credits with much of the work behind this elemental study. She hopes that better understanding the iron-organic carbon stabilizing mechanism could "eventually lead to new ways of increasing the rate of organic carbon burial in sediments."
More information: www.nature.com/nature/journal/ … ull/nature10855.html
Provided by Concordia University