To make better fuel cells, study the defects
Engineers trying to improve fuel-cell catalysts may be looking in the wrong place, according to new research at Cornell.
There is growing interest in forming the catalysts that break down fuel to generate electricity into nanoparticles. Nanoparticles provide a larger surface area to speed reactions, and in some cases, materials that are not catalytic in bulk become so at the nanoscale.
These nanoparticles, typically just a few tens of nanometers (nm) wide, are not neat little spheres, but rather jagged chunks, like microscale gravel, and researchers have found that they can correlate catalytic activity with information about the number and type of their surface facets. But they may be looking at the forest and ignoring the trees.
"People measure the activity of a sample and then try to understand by using facet information," said Peng Chen, associate professor of chemistry and chemical biology. "The message we want to deliver is that surface defects [on the facets] dominate the catalysis."
Chen's research is reported Feb. 19 in the online edition of the journal Nature Nanotechnology.
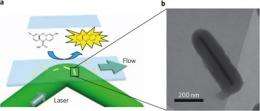
Instead of particles, Chen's research group studied catalytic events on gold "nanorods" up to 700 nm long, effectively letting them see how activity varies over a single facet. Gold acts as a catalyst to convert a chemical called Amplex Red into resorufin, which is fluorescent.
Each time a catalytic event occurs, the newly created molecule of resorufin emits a flash of light that is detected by a digital camera looking through a microscope. A flash typically appears as several pixels, and additional computer processing averages their brightness to pinpoint the actual event to within a few nanometers. The researchers call the technique "super-resolution microscopy." After flooding a field of nanorods with a solution of Amplex Red, they made a "movie" with one frame every 25 milliseconds.
The researchers found more catalytic events near the middle of a rod, tapering off toward the ends and a jump back up at the ends. They also found variation in the amount of activity from one rod to another, even though all the rods have the same types of facets.
To explain the results, they proposed that activity is higher in areas where there are more surface defects. The nanorods are made by growing gold crystals from a small "seed" crystal, growing outward from the center to the ends, Chen explained, and more defects form at the beginning of the process.
"Knowledge of the surface facets ... is insufficient to predict reactivity," the researchers said in their paper. "Surface defects … can also play a dominant role."
The findings with a gold catalyst and fluorescent molecules should be equally applicable to other catalysts, including those used in fuel cells and for pollution remediation, Chen said.
Provided by Cornell University

















