Scientists map one of life's molecular mysteries
All living organisms are made up of cells, behind these intricate life forms lie complex cellular processes that allow our bodies to function. Researchers working on protein secretion — a fundamental process in biology — have revealed how protein channels in the membrane are activated by special signals contained in proteins destined for secretion. The results help explain the underlying mechanism responsible for the release of proteins such as hormones and antibodies into the blood stream.
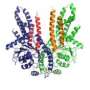
The findings, published today in the inaugural issue of Cell Reports, represent a major step forward in cell biology. Until now, scientists have been frustrated by not knowing the architecture of the protein transport machinery when engaged by cargo. However, the team led by researchers from the University of Bristol as part of an international collaboration, has successfully produced and visualised such a complex.
All cells are surrounded by membranes, made up from a double layer of fatty molecules called phospholipids. These act as an ideal 'skin', keeping the cell's insides in. In the absence of other components these fatty molecules act as barriers, preventing the necessary rapid exchange of nutrients and waste products, and of larger molecules like proteins, between the environment and the cell interior. However, such movement is required for many proteins to perform their biological functions either within the membrane or the outside.
To overcome this problem, biological membranes contain a number of translocation systems that enable proteins and other useful substances to pass across the phospholipid barrier. In the case of proteins, those destined for transport are recognised by translocation systems via signals embedded in the sequence of amino acids from which they are constructed. Correct passage through or across the membrane is critical in ensuring that cells complete their lifecycle and fulfill their function.
Using electron microscopy and results from X-ray crystallography, Ian Collinson, Professor of Biochemistry at the University, and his team have described the structure of the ubiquitous Sec-complex associated with a bona fide mimic of a pre-secretory protein in the native environment of the membrane. These results reveal how the binding of the signal sequence unlocks the Sec-complex prior to channel opening and pre-protein transport.
Professor Collinson from the University's School of Biochemistry, said: "These findings are important as they address outstanding questions in one of the central pillars of biology, a process essential in every cell in every organism. The results may suggest ways in which the process can be corrupted in order to manage specific disease states or bacteria infections."
More information: The paper, entitled Structure of the SecY complex unlocked by a pre-protein mimic, by Hizlan, D, Robson, A, Whitehouse, S, Gold, V.A, Vonck, J, Mills, D.J, Kühlbrandt, W. and Collinson, I. is published in Cell Reports.
Provided by University of Bristol