January 21, 2012 weblog
Iridescence and superhydrophobicity combined on one surface
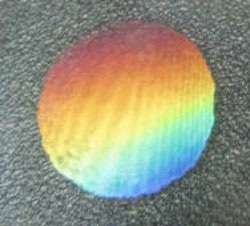
(PhysOrg.com) -- Scientists have combined two properties on a single piece of graphene oxide that don’t usually go together: iridescence (resulting in a rainbow-hued appearance) and superhydrophobicity (causing low-contact water droplets to strongly adhere to the surface). The engineered surface could have applications in liquid transportation and analysis, and due to graphene’s good electronic properties, possibly even in future electronic devices.
The researchers, Jian-Nan Wang and coauthors from Jilin University in Changchun, China, achieved these properties by creating a microscopic texture on the graphene oxide’s surface. By shining two laser beams on the surface, they created an interference pattern that burned tiny grooves into the material. The surface immediately took on an iridescent appearance, shimmering like a butterfly’s wing. The scientists explained that the tiny grooves, which form highly ordered periodic structures, act as diffraction gratings that split white light into its various colored wavelengths.
The researchers discovered that the surface effects also caused the graphene oxide to exhibit highly adhesive superhydrophobicity. When they poured water on the surface, the water merged into nearly spherical droplets. Although the droplets are barely in contact with the graphene oxide, they adhere to the surface, not detaching even when the surface is held upside down. The researchers attributed this phenomenon to the surface’s microscopic unevenness and a decrease in surface energy caused during laser irradiation, which removes some hydrophilic oxygen groups.
The researchers predict that the combination of these two properties on one surface could have applications in microfluidic devices, where a superhydrophobic surface is useful for transporting small amounts of liquid in a controlled way. Graphene oxide might also be used as a biocompatible surface for growing cells, which requires controlled wetting.
The iridescence might be used for color-coding different states in microfluidic devices, as well as decorating for aesthetic purposes. Also, by altering the optical properties of graphene-based devices, it may open doors to new optical or electronic devices.
More information:
Jian-Nan Wang, et al. “Biomimetic Graphene Surfaces with Superhydrophobicity and Iridescence.” Chemistry - An Asian Journal. DOI: 10.1002/asia.201100882
via: Chemistry World
© 2011 PhysOrg.com


















