Hot molecule explains cold chemistry
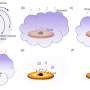
(PhysOrg.com) -- Surprisingly, hydrogen cyanide and its far more energetic isomer, hydrogen isocyanide, are present in almost equal amounts in cold interstellar gas clouds. Scientists from the Max Planck Institute for Nuclear Physics have succeeded in explaining how this happens through experiments carried out in the Heidelberg ion storage ring. During interstellar synthesis hydrogen cyanide forms as a hot hybrid from which the two isomers evolve in about equal quantities.
When stars form from cold gas clouds, the clouds already contain many molecules consisting of important basic elements such as hydrogen, carbon, oxygen and sulfur. Sensitive new observatories enable fingerprints of many of these molecules to be identified in the light and the radio emission of the gas clouds. These spectroscopic observations reveal that the atoms in the interstellar molecules do not always arrange in the energetically most advantageous way.
Some of the observed compounds are found in related forms (isomers) which can arise when individual atoms within a molecule interchange their positions. But such position changes go at the cost of considerable energy, equivalent to temperatures of several thousand degrees.
One of these molecules is hydrogen cyanide or prussic acid (HCN – the hydrogen atom is bound to the carbon atom), whose much more energy-rich isomer hydrogen isocyanide (HNC – the hydrogen atom is bound to the nitrogen atom) is as abundant as hydrogen cyanide itself, although the latter should largely prevail at the low temperatures in open space.
Researchers long suspected that these often highly energetic isomers are finally a consequence of the ionizing radiation that permeates space. In fact, a symmetrical precursor, the HCNH+ ion, forms through an intricate chain of reactions. Later, this HCNH+ ion can encounter an electron, by which it is neutralized and dissociated into fragments, releasing energy. This way, both isomers can be formed.
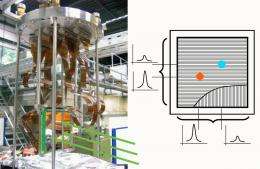
Scientists at the Max Planck Institute for Nuclear Physics have now accurately measured the properties of this elementary dissociation reaction in the laboratory – under conditions very similar to those found in interstellar clouds. In the Heidelberg ion storage ring, they made electrons and DCND+ ions (variants of HCNH+ with heavy hydrogen, D = deuterium) collide one by one and, moreover, at very low collision energies; in interstellar clouds, these energies correspond to a temperature around minus 260 degrees Celsius.
Using a recently developed large-area detector, the researchers measured both positions and particle masses of the fragments D and DCN or DNC; only by this instrument it could be ensured that dissociation into exactly these two particles was selectively observed in the experiment. This method is still unable to distinguish between the two isomers of the product molecule; but it offers the unique advantage that the kinetic energy of the fragments can be determined accurately.
Here the researchers observed kinetic energy releases that were far smaller than expected. The missing amount of energy can only be contained inside the product molecule – thus, as predicted by some theoreticians, the molecule is extremely “hot” corresponding to its high internal excitation energy. This implies, however, that in this strongly vibrating product of a cold reaction, atoms can still change positions easily and frequently.
The molecule formed in interstellar gas clouds can therefore assume both geometric forms while it gradually emits its high internal energy into the environment – like a slowly dimming light bulb. The energy-rich isomer arises here in about half of all cases. Hence – via a long detour, now experimentally demonstrated in the laboratory – the presence of this isomer in interstellar molecular clouds reflects its production mechanism, ultimately owing to ionizing radiation.
More information: Mario B. Mendes et al., Cold electron reactions producing the energetic isomer of hydrogen cyanide in interstellar clouds, Astrophysical Journal Letters, January 20, 2012
Journal information: Astrophysical Journal Letters
Provided by Max-Planck-Gesellschaft