Flexible adult stem cells, right there in your eye
In the future, patients in need of perfectly matched neural stem cells may not need to look any further than their own eyes. Researchers reporting in the January issue of Cell Stem Cell, a Cell Press publication, have identified adult stem cells of the central nervous system in a single layer of cells at the back of the eye.
That cell layer, known as the retinal pigment epithelium (RPE), underlies and supports photoreceptors in the light-sensitive retina. Without it, photoreceptors and vision are lost. The new study shows that the RPE also harbors self-renewing stem cells that can wake up to produce actively growing cultures when placed under the right conditions. They can also be coaxed into forming other cell types.
"You can get these cells from a 99-year-old," said Sally Temple of the Neural Stem Cell Institute in Rensselaer, New York. "These cells are laid down in the embryo and can remain dormant for 100 years. Yet you can pull them out and put them in culture and they begin dividing. It is kind of mind boggling."
Temple's group got the RPE-derived stem cells they describe from the eyes of donors in the hours immediately after their deaths. But the cells can also be isolated from the fluid that surrounds the retina at the back of the eye, which means they are accessible in living people as well.
"You can literally go in and poke a needle in the eye and get these cells from the subretinal space," she says. "It sounds awful, but retinal surgeons do it every day." By comparison, access to most other neural stem cell populations would require major surgery.
Temple said they were curious about the proliferative potential of the RPE given that the tissue is known to be capable of regenerating entire retinas in salamanders. But that plasticity in adulthood had seemed to be lost in mice and chicks. Still, "given the evolutionary evidence, we thought it was worth revisiting," she said.
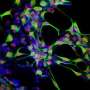
They placed RPE tissue taken from 22-year-old to 99-year-old cadavers into many culture conditions to see what they could make the cells do. They found one set of conditions that got the cells dividing. Not all of the RPE cells have this regenerative potential, but perhaps 10 percent of them do.
Further work showed that the cells are multipotent, which means that they can form different cell types, though the researchers admit there is more to do to fully explore the cells' differentiation capacity.
There are other implications as well. For example, these cells may explain diseases in which other tissue types show up in the eye. Their presence also suggests that there might be some way to stimulate controlled repair of the eye in the millions of people who suffer from age-related macular degeneration.
"I think it might be possible," Temple said.
More information: Salero et al.: "Adult Human RPE Can Be Activated into a Multipotent Stem Cell that Produces Mesenchymal Derivatives." Cell Stem Cell January 6, 2012 print issue. DOI:10.1016/j.stem.2011.11.018
Abstract
The retinal pigment epithelium (RPE) is a monolayer of cells underlying and supporting the neural retina. It begins as a plastic tissue, capable, in some species, of generating lens and retina, but differentiates early in development and remains normally nonproliferative throughout life. Here we show that a subpopulation of adult human RPE cells can be activated in vitro to a self-renewing cell, the retinal pigment epithelial stem cell (RPESC) that loses RPE markers, proliferates extensively, and can redifferentiate into stable cobblestone RPE monolayers. Clonal studies demonstrate that RPESCs are multipotent and in defined conditions can generate both neural and mesenchymal progeny. This plasticity may explain human pathologies in which mesenchymal fates are seen in the eye, for example in proliferative vitroretinopathy (PVR) and phthisis bulbi. This study establishes the RPESC as an accessible, human CNS-derived multipotent stem cell, useful for the study of fate choice, replacement therapy, and disease modeling.
Journal information: Cell Stem Cell
Provided by Cell Press