First complete 3D visualization of vitamin D receptor
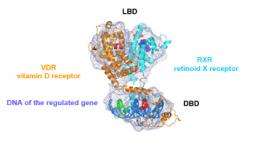
For the first time, a team from the Institut de Génétique et de Biologie Moléculaire et Cellulaire, France, has obtained a high-resolution, full 3D image of a small but vital molecule locked up within our cells: the vitamin D receptor (VDR). Published on 18 January 2012 in The EMBO Journal, this study provides key information on the 3D structure and action mechanism of the receptor at the molecular scale. This data is crucial to pharmaceutical research, since VDR is involved in numerous diseases such as cancer, rickets and type 1-diabetes.
Member of what biologists call “the large family of nuclear receptors” - or proteins active in the nucleus of cells - along with “steroidal” receptors (sexual hormone receptors, etc.), the vitamin D receptor (VDR) plays an important role in regulating the expression of genes involved in various vital biological functions (cell growth, bone mineralization, etc.).
Until now, researchers had only been able to study two parts of this receptor at close range: the DNA interaction region and the vitamin D binding domain. These two parts had been produced in the laboratory and their structure studied individually using crystallography techniques. However, this method does not lend itself to mapping VDR in its entirety since the receptor is difficult to crystallize.
To meet this challenge, which has called upon the resources of many researchers around the world for more than 15 years, the teams led by Bruno Klaholz and Dino Moras, both CNRS senior researchers at IGBMC, resorted to an innovative technique: cryo-electron microscopy (cryo-EM), which requires a next-generation “high-resolution” electron microscope. This technological marvel makes it possible to observe biological objects at the molecular and even atomic scale. In France, the first such EM was installed at IGBMC in 2008. Before this work was carried out, it was widely assumed that studying VDR with cryo-EM was impossible. In fact, until now, the smallest molecules observed using this technique weighed more than 300 kilodaltons (kDa), or even several thousand kDa, in other words much more than the VDR, which weighs 100 kDa and measures a mere 10 nm (10 x 10-9 m).
In concrete terms, Klaholz and his colleagues produced large quantities of human VDR in Escherichia coli bacteria (one of the most widely used models in biology to produce proteins) in the laboratory. They then isolated the receptor in a physiological solution containing water and a little salt. The sample containing the VDR was then flash-frozen by immersion in liquid ethane, allowing extremely rapid cooling (within a fraction of a second the sample drops from 25°C to around -184°C). Finally, the team had to take 20,000 photographs of VDR particles in different orientations, using the microscope. Once aligned and combined via a computer program, these images finally provided a full 3D reconstruction of the VDR.
This image supplies new information on how the receptor works. It reveals that the VDR and its partner RXR (retinoid X receptor, a vitamin A derivative) form an open architecture, with the vitamin D binding domain almost perpendicular to the DNA binding domain. This structure suggests cooperation between both domains, which could act together to induce highly-precise regulation of target gene expression.
This pioneering work opens the way to the elucidation of several other, still poorly understood vital nuclear receptors. In particular, biologists are now looking at using cryo-EM to reveal the structure of steroidal receptors.
More information: Structure of the full human RXR/VDR nuclear receptor heterodimer complex with its DR3 target DNA. Igor Orlov, Natacha Rochel, Dino Moras and Bruno Klaholz. The EMBO Journal. 18 January 2012.
Provided by CNRS