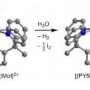
Making molecular hydrogen more efficiently
(PhysOrg.com) -- When it comes to the industrial production of chemicals, often the most indispensable element is one that you can't see, smell, or even taste. It's hydrogen, the lightest element of all.
Researchers at the U.S. Department of Energy's (DOE) Argonne National Laboratory have developed an extraordinarily efficient two-step process that electrolyzes, or separates, hydrogen atoms from water molecules before combining them to make molecular hydrogen (H2), which can be used in any number of applications from fuel cells to industrial processing.
Easier routes to the generation of hydrogen have long been a target of scientists and engineers, principally because the process to create the gas requires a great deal of energy. Approximately 2 percent of all electric power generated in the United States is dedicated to the production of molecular hydrogen, so scientists and engineers are searching for any way to cut that figure. "People understand that once you have hydrogen you can extract a lot of energy from it, but they don't realize just how hard it is to generate that hydrogen in the first place," said Nenad Markovic, an Argonne senior chemist who led the research.
While a great deal of hydrogen is created by reforming natural gas at high temperatures, that process creates carbon-dioxide emissions. "Water electrolyzers are by far the cleanest way of producing hydrogen," Markovic said. "The method we've devised combines the capabilities of two of the best materials known for water-based electrolysis."
Most previous experiments in water-based electrolysis rely on special metals, like platinum, to adsorb and recombine reactive hydrogen intermediates into stable molecular hydrogen. Markovic's research focuses on the previous step, which involves improving the efficiency by which an incoming water molecule would disassociate into its fundamental components. To do this, Markovic and his colleagues added clusters of a metallic complex known as nickel-hydroxide—Ni(OH)2. Attached to a platinum framework, the clusters tore apart the water molecules, allowing for the freed hydrogen to be catalyzed by the platinum.
"One of the most important points of this experiment is that we're combining two materials with very different benefits," said Markovic. "The advantage of using both oxides and metals in conjunction dramatically improves the catalytic efficiency of the whole system."
According to Argonne materials scientist George Crabtree, who helped to initiate the establishment of Argonne's energy conversion program, the researchers' success is attributable to their ability to work on what are known as "single-crystal" systems—defect-free materials that allow scientists to accurately predict how certain materials will behave at the atomic level. "We have not only increased catalytic activity by a factor of 10, but also now understand how each part of the system works. By scaling up from the single crystal to a real-world catalyst, this work illustrates how fundamental understanding leads quickly to innovative new technologies."
This work, supported by the DOE Office of Science, is reported in the December 2 issue of Science.
Provided by Argonne National Laboratory