Glass that cleans itself
Eyeglasses need never again to be cleaned, and dirty windscreens are a thing of the past! Researchers at the Max Planck Institute for Polymer Research in Mainz and the Technical University Darmstadt are now much closer to achieving this goal. They have used candle soot to produce a transparent superamphiphobic coating made of glass. Oil and water both roll off this coating, leaving absolutely nothing behind. Something that even held true when the researchers damaged the layer with sandblasting. The material owes this property to its nanostructure. Surfaces sealed in this way could find use anywhere where contamination or even a film of water is either harmful or just simply a nuisance.
Doris Vollmer hates it that her eyeglasses always get dirty so quickly. However, the scientist, who heads a research group at the Max Planck Institute for Polymer Research, is looking for a solution to the problem - and she and her team are now a good deal closer to finding one. A transparent coating that is very good at repelling water and oil, as is now being presented by the Mainz-based researchers, could not only keep water and dirt away from the lenses in glasses and car windscreens, but also, for example, from the glass facades of skyscrapers. It could also prevent residues of blood or contaminated liquids on medical equipment.
The coating essentially consists of an extremely simple material: silica, the main constituent of all glass. The researchers coated this with a fluorinated silicon compound, which already makes the surface water and oil repellent, like a non-stick frying pan. The really clever part is the structure of the coating, however. This is what makes the glass super water repellent and super oil repellent. In a frying pan with this type of coating, water and oil would simply roll around in the form of drops. The structure of the layer resembles a sponge-like labyrinth of completely unordered pores, which is made up of tiny spheres.
Soot from the candle flame as model for the porous glass structure
“The rounded surfaces cannot be wet even by low-viscosity oils, although this would be energetically most favourable,” says Doris Vollmer. This is because the liquids that wet even fluorinated surfaces would have to be pressed over these spheres, which measure around 60 nanometres (one nanometre corresponds to one millionth of a millimetre), in order to form a film on the surface. This requires too much energy.
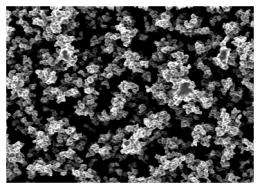
Such a coating would be ideal for numerous applications, not least because it is so easy to produce. “We can even produce it in jam jars,” says Doris Vollmer. And the soot from a candle flame, from which the researchers made something akin to a glass imprint, served as the model for the porous structure of the spheres. The researchers began by holding a glass slide in a flame so that the soot particles, which measure around 40 nanometres in diameter, formed a sponge-like structure on the glass. The next step was to coat it with silica in a glass vessel – even a jam jar would do – by vapour depositing a volatile organic silicon compound and ammonia onto the soot deposit. When they subsequently heated the material, the soot decomposed. The next step was to vapour deposit a fluorinated silicon compound as well onto the hollow silica structure.
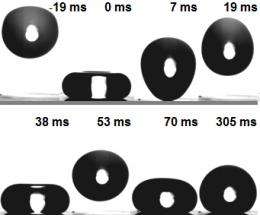
They then attempted to wet this coating with different liquids. However, they didn’t succeed, even when they let hexadecane drip from a great height onto it; in a non-stick frying pan, hexadecane spreads out like water in a washbasin. “Initially, a drop of the oil penetrated into the sponge-like structure, but then bounced back like a rubber ball,” explains Doris Vollmer. Although a portion of the liquid remained in the pores and wet the material, when most of the drop returned to the surface at a slower speed after bouncing up, it drew the small amount of the hexane that had remained out of the glass pores again. Finally, the reunited drop remained lying on the surface like a ball (see video). The researchers in Mainz tested the superamphiphobic layer with a total of seven liquids and found that none was sucked up by the glass sponge.
Systematic research for self-cleaning coating
“As the material repels water and oil so well, it would be suitable as a self-cleaning coating for a large number of applications,” says Hans-Jürgen Butt, Departmental Director at the Mainz-based Max Planck Institute where Doris Vollmer works with her group. And even if a portion of the layer was removed, the glass structure remained superamphiphobic. This is because its internal structure is the same as its structure on the surface. It only loses its self-cleaning properties when the layer becomes thinner than one micrometre. And this is precisely what would happen quite soon in practice, even if a self-cleaning sponge structure several micrometres thick was used to coat the lenses of eyeglasses or a windowpane. When the researchers let sand trickle onto the delicate glass structure, the coating was worn away quite quickly. “In a next step, we would therefore like to develop a layer that is superamphiphobic, with better mechanical stability,” says Doris Vollmer.
Through the aid of such coatings the researchers want to find out more about the factors that determine how well a material repels water and oil. “We still don’t know this relationship in detail,” says Hans-Jürgen Butt. “The search for superamphiphobic materials is therefore more or less a case of trial and error.” As soon as the researchers have achieved a systematic understanding of why a liquid wets a surface or not, industrial companies will be able to specifically develop self-cleaning coatings for applications in architecture, optics and medicine.
More information: Xu Deng, et al. Transformation of black candle soot into a transparent robust superamphiphobic coating, Science Express, December 1, 2011.
Provided by Max-Planck-Gesellschaft
















