How old yeast cells send off their daughter cells without the baggage of old age
The accumulation of damaged protein is a hallmark of aging that not even the humble baker's yeast can escape. Yet, aged yeast cells spawn off youthful daughter cells without any of the telltale protein clumps. Now, researchers at the Stowers Institute for Medical Research may have found an explanation for the observed asymmetrical distribution of damaged proteins between mothers and their youthful daughters.
Reporting in the Nov. 23, 2011, issue of Cell the research team, led by Stowers investigator Rong Li, Ph.D., proposes that the limited mobility of clumps of damaged proteins and yeast cells' geometry -- the narrowness of the connection (bud neck) between the mother and the daughter before their separation, in particular -- are sufficient to ensure that protein aggregates accumulated during the normal aging process are retained in the mother cell during cell division.
"Harmful protein aggregates had recently been thought to be sent back into the mother cell via a directed transport system," says Li. "Our model suggests that no active shuttle mechanism may be necessary to help with the asymmetric segregation of protein aggregates during yeast cell divisions."
In the budding yeast Saccharomyces cerevisae -- an important model organisms used in aging research -- lifespan can be defined by the number of daughter cells a mother has produced, as opposed to by calendar time, a process known as replicative aging. Daughter cells reset their clock and start counting the number of cell division they have undergone from scratch.
The transition from youth to old age is accompanied by metabolic changes and the accumulation of damage as a result of wear and tear. A central question in aging research is the nature of the damage that contributes to aging and how old mother cells avoid passing on these aging determinants to their daughters.
One factor that is known to correlate with replicative age is the buildup of aggregates formed by damaged proteins. "These proteins are preferentially retained by the mother during bud formation and cell division," explains Li. "A better understanding of replicative aging of a cell population based on asymmetric cell divisions may provide insights into how higher organisms maintain a population of "youthful" stem cells with high proliferative potential during aging."
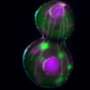
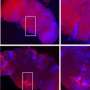
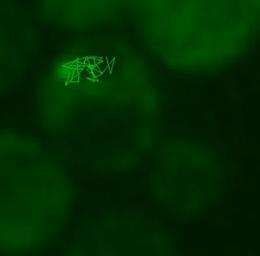
To learn more about the movement and fate of damaged proteins in dividing yeast cells, graduate student and first author Chuankai Zhou with help from Amr Eldakak, Ph.D, a postdoctoral research associate in the Li laboratory, added a green fluorescent tag to Hsp104p, a protein known to modify and dissolve protein aggregates by unfolding and refolding proteins. Zhou then used live-cell imaging to record the movements of thousands of protein aggregates induced by heat in three dimensions.
"Most movements were confined within the bud or the mother but we did see a few movements from bud to mother and vice versa," says Zhou. "Overall though, we couldn't detect any directionality in the movements of the aggregates." In order to rigorously characterize the movement of the protein aggregates, Zhou collaborated with Stowers Research advisors Brian Slaughter, Ph.D., and Jay Unruh, Ph.D., and used particle tracking and computational analysis to show that the aggregate movement is best described as 'random walk'.
Time-lapse movies also revealed that, over time, heat shock-induced aggregates cleared from all buds and their numbers plummeted in mother cells. When Zhou introduced a mutation into Hsp104p that does not affect Hsp104p's ability to bind to protein aggregates but disrupts its refolding activity, aggregates no longer cleared from neither mother nor daughter cell. "It told us that heat-induced aggregates dissolved with the help of Hsp104p," explains Zhou.
Zhou then turned his attention to naturally occurring protein aggregates, which are the result of oxidative damage in cells of older replicative age. He found that these protein clumps followed the same random walk pattern but didn't dissolve over time. However, these aggregates appeared to move within the confines of the mother without escaping into the bud.
With the help of Stowers research advisor Boris Rubinstein, the team used 3D numerical simulations as well as a 1D analytical model to show that the limited, random mobility of the aggregates was sufficient to explain their preferential retention in the mother, and that the narrow opening of the bud neck further helps trapping the aggregates within the mother prior to cell division.
Provided by Stowers Institute for Medical Research